NEURONETICS, INC. DISTRIBUTION AGREEMENT
Exhibit 10.1
This Distribution Agreement (this “Agreement”) is made and entered into this 12th day of October 2017 (the “Effective Date”) by and between Neuronetics, Inc., a Delaware corporation having its principal offices at ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇, ▇▇▇ (“Company”), and Teijin Pharma Limited, a Japanese company having its principal offices at ▇-▇, ▇▇▇▇▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇ (“Distributor”). Each of Company and Distributor are sometimes referred to individually in this Agreement as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, Company desires to appoint Distributor as a distributor of the Products (as defined below) in the Territory (as defined below), subject to the terms and conditions of this Agreement; and
WHEREAS, Distributor desires to accept such appointment.
NOW, THEREFORE, Company and Distributor, intending to be legally bound, agree as follows:
1. Definitions.
1.1 The following terms shall be defined as follows:
1.1.1 “1st Reimbursement Approval” means the initial Reimbursement Approval issued by MHLW.
1.1.2 “1st Qualifying Approval” means (a) if the Product Approval and/or the 1st Reimbursement Approval requires a physician to obtain a training certification in respect of use of the System from a person, other than Distributor, its Affiliates or persons acting on behalf of Distributor or its Affiliates, in order to be permitted to use the System to treat patients on a reimbursed basis, then the first time that any physician in the Territory is granted such certification or (b) if no such training certification is required by the Product Approval or the 1st Reimbursement Approval, then the 1st Reimbursement Approval.
1.1.3 “1st Reimbursement Revision Approval” means the next permanent Reimbursement Approval issued by MHLW (honshusai) after the 1st Reimbursement Approval.
1.1.4 “Affiliate” means, with respect to a person, any individual, group, corporation, limited liability company, partnership, joint venture, association, trust and any other legal entity directly or indirectly Controlled by, Controlling, or under common Control with such person. The term “Control” of a legal entity means the possession, direct or indirect, of the power to: (a) vote more than fifty percent of the voting stock of such legal entity; or (b) direct or cause the direction of the management or policies of such legal entity, whether through the ownership of securities or partnership or other ownership interests, by contract or otherwise.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
1.
1.1.5 “Anti-Corruption Laws” has the meaning set forth in Section 5.5.1.
1.1.6 “Applicable Law” means all Laws, including cGMP, relevant to each Party’s obligations under this Agreement, including, without limitation, any rules, regulations, guidelines or other requirements of the FDA and any other Governmental Authority with respect to the manufacture of the Products and rules, regulations, guidelines or other requirements of the MHLW applicable to the promotion, marketing, distribution and sale of the Products in the Territory.
1.1.7 “Baseline Year” means the Fiscal Year in which either of the Parties or their respective Affiliates commences sales of Home Use Devices in the Territory.
1.1.8 “Certificate of Conformance” means that certificate to be delivered by Company’s contract manufacturer to Distributor in the form attached as Schedule L.
1.1.9 “Change of Control” means with respect to a Party (a) a merger or consolidation of such Party with a third party which results in the voting securities of Company outstanding immediately prior thereto ceasing to represent at least fifty percent (50%) of the combined voting power of the surviving entity immediately after such merger or consolidation, (b) except in the case of a bona fide equity financing in which such Party issues new shares of its capital stock, a transaction or series of related transactions in which a third party, together with its Affiliates, becomes the beneficial owner of fifty percent (50%) or more of the combined voting power of the outstanding securities of Company, or (c) the sale or other transfer to a third party of all or substantially all of such Party’s business to which the subject matter of this Agreement relates.
1.1.10 “Code” has the meaning set forth in Section 5.6.
1.1.11 “Company MHLW Lead Period” means the period from the date of the 1st Reimbursement Approval until and including the date of the 1st Reimbursement Revision Approval.
1.1.12 “Company Trademarks” means (a) the English language trademarks listed on Schedule D and (b) the katakana trademarks listed on Schedule D.
1.1.13 “Company’s Agent” means Vorpal or such other company as Company designates from time to time by sending notice to Distributor.
1.1.14 “Competitive Product” means [*]
1.1.15 “Confidential Information” has the meaning set forth in Section 8.1.
1.1.16 “cGMP” means the current good manufacturing practices applicable to the manufacture of the Product under this Agreement as defined in the U.S. Current Good Manufacturing Practices, 21 C.F.R. Part 820, and the equivalent Laws in the Territory, each as may be amended and applicable from time to time.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
2.
1.1.17 “Default Notice” shall have the meaning set forth in Section 6.3.
1.1.18 “Defect” means, in respect of a Product, a manufacturing defect, a failure to meet or operate in accordance with the applicable Specifications or a failure to have been manufactured in accordance with Applicable Law including cGMP, and, in the case of the Software, a failure to operate in substantial compliance with the Software Documentation, and “Defective” shall be construed accordingly.
1.1.19 “Defective Product” means a Product with a Defect.
1.1.20 “Designated Marketing Authorization Holder” or “DMAH” means the agent approved by the MHLW to act as the MAH in accordance with and as defined in the Law on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics.
1.1.21 “Disclosing Party” has the meaning set forth in Section 8.1.
1.1.22 “Distributor 1st MHLW Lead Election” has the meaning set forth in Section 4.1.2.
1.1.23 “Distributor 1st MHLW Lead Period” means the [*] period following the date of Distributor’s written notice making the Distributor 1st MHLW Lead Election.
1.1.24 “Distributor 2nd MHLW Lead Election” has the meaning set forth in Section 4.1.4.
1.1.25 “Distributor 2nd MHLW Lead Period” means the [*] period following the date of the 1st Reimbursement Revision Approval.
1.1.26 “Distributor Approvals” has the meaning set forth in Section 4.2.
1.1.27 “Distributor Quality Plan” means the separate Distributor Quality Plan between the Parties, as amended by the Parties from time to time. The current Distributor Quality Plan is document number [*]
1.1.28 “Distributor Trademarks” has the meaning set forth in Section 7.2.2
1.1.29 “Documentation” shall mean any and all information in written, graphic, electronic or machine-readable form relating to use or operation of the System, including but not limited to, the System user manual and instructions for use, installation and service of the System provided by Company to Distributor pursuant to this Agreement.
1.1.30 “Dollar,” “Dollars,” “U.S. Dollars” and the symbol “$” shall mean lawful money of the United States of America.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
3.
1.1.31 [*]
1.1.32 “FDA” means the United States Food and Drug Administration or its successor.
1.1.33 “Fiscal Year” means the period commencing on April 1 of each calendar year and ending on March 31 of the subsequent calendar year.
1.1.34 “Fixed Transfer Price Period” has the meaning set forth in Section 3.5.1.
1.1.35 “Force Majeure” has the meaning set forth in Section 14.13.
1.1.36 “Foreign Manufacturer Accreditation” means the license issued by M1-ILW to a manufacturer of medical devices located outside of Japan for import and sale of such medical devices in Japan as specified in Article 13-3 of the Pharmaceuticals Affairs Law of Japan.
1.1.37 “Governmental Authority” means any multinational, national, federal, prefectural, state, local, municipal or other governmental authority of any nature (including any governmental division, subdivision, department, agency, bureau, branch, office, commission, council, court or other tribunal), in each case, having jurisdiction over the applicable subject matter.
1.1.38 “Government Official” has the meaning set forth in Section 5.5.1.
1.1.39 “[*] Baseline” means the Dollar value of Distributor’s purchases of Products from Company during the first Fiscal Year after the Baseline Year.
1.1.40 “[*] Credit” has the meaning set forth in Section 3.12.
1.1.41 “[*] Device” means any transcranial magnetic stimulation device that is, or is intended to be, marketed for use by [*] for the treatment of Major Depressive Disorder.
1.1.42 “[*] Minimum” means the higher of (a) the Dollar value of purchases of Products by Distributor from Company during the Baseline Year or (b) the Dollar value of purchases of Products by Distributor from Company during the full Fiscal Year prior to the Baseline Year.]
1.1.43 “Indemnification Claim Notice” has the meaning set forth in Section 10.2.1.
1.1.44 “Indemnified Party” has the meaning set forth in Section 10.2.1.
1.1.45 “Indemnifying Party” has the meaning set forth in Section 10.2.1.
1.1.46 “Indemnitee” and “Indemnitees” has the meaning set forth in Section 10.2.1.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
4.
1.1.47 “Initial Milestone Payment 2” means Milestone Payment 2 calculated using the Reimbursement Rate set in the 1st Reimbursement Approval.
1.1.48 “Initial Payment” has the meaning set forth in Section 3.1.1.
1.1.49 “Initial Period” has the meaning set forth in Section 3.4.2.
1.1.50 “Initial Sales Forecast” has the meaning set forth in Section 3.4.2.
1.1.51 “Initial Term” has the meaning set forth in Section 12.1.
1.1.52 “Initial Transfer Price” has the meaning set forth in Section 3.5.1.
1.1.53 “Insolvent Party” has the meaning set forth in Section 12.12.
1.1.54 “[*]” means [*] that the Company and Distributor will develop pursuant to Section 6.1 [*]
1.1.55 “Laws” means all laws, statutes, rules, regulations, directives, decisions and ordinances of any Governmental Authority.
1.1.56 “Limited License” has the meaning set forth in Section 7.3.1.
1.1.57 “Losses” has the meaning set forth in Section 10.1.1.
1.1.58 “MAH” means Marketing Authorization Holder.
1.1.59 “Major Depressive Disorder” has the meaning set forth in ICD-9 §§ 296.X.
1.1.60 “Marketing Materials” has the meaning set forth in Section 7.5.
1.1.61 “MHLW” means the Japanese Ministry of Health, Labour and Welfare and any successor thereto.
1.1.62 “Milestone Payment 1” has the meaning set forth in Section 3.1.2.
1.1.63 “Milestone Payment 2” has the meaning set forth in Section 3.1.3.
1.1.64 “Minimum Purchase Requirement” has the meaning set forth in Section 3.4.1.
1.1.65 “Minimum Terms and Conditions of Sale” has the meaning set forth in Section 7.3.4.
1.1.66 “NeuroStar Product” means the NeuroStar TMS Therapy® System, Item Number ▇▇-▇▇▇▇▇-▇▇▇ as listed in the Product Catalog and set forth in Schedule A. For the avoidance of doubt, the NeuroStar Product does not include the TrakStar Computer and peripherals, the NeuroStar Treatment Packs or the other items included in the NeuroStar Starter Package.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
5.
1.1.67 “NeuroStar Starter Package” means a bundled package of the Products set forth in Schedule A together with the Limited License.
1.1.68 “NeuroStar Treatment Packs” means alignment and hygiene barrier consumables used in conjunction with NeuroStar Treatment Sessions, Item Number ▇▇-▇▇▇▇▇-▇▇▇ as listed in the Product Catalog.
1.1.69 “NeuroStar Treatment Sessions” or “NSTS” means a single treatment session delivered by the NeuroStar Product to a patient, comprised of a maximum of 5,000 magnetic pulses and delivered in accordance with the treatment parameters specified by the prescribing physician. The treatment parameters include but are not limited to number of pulses, stimulation time, stimulation frequency, interval and coil orientation.
1.1.70 “New Sales Forecast” has the meaning set forth in Section 3.4.2.
1.1.71 “[*] Development Plan” has the meaning set forth in Section 6.1.
1.1.72 “Out of Box Failure” means at the time of installation at the customer’s facility of a NeuroStar Starter Package (a) non-conformance to the Certificate of Conformance provided by Company’s contract manufacturer for such NeuroStar Starter Package; or (b) a failure of the NeuroStar Starter Package to pass the visual inspection procedure set forth in Schedule E or any of the criteria or items set forth in the NeuroStar OUS Installation Record [*], after Distributor or its Technical Support Company follows all installation and troubleshooting procedures in the NeuroStar Distributor Service Manual [*], other than any such non-conformance or failure caused by Distributor or its Technical Support Company after Company’s delivery of such NeuroStar Starter Package to Distributor.
1.1.73 “Order Forecast” has the meaning set forth in Section 3.2.2.
1.1.74 “PMDA” means the Pharmaceuticals and Medical Devices Agency and any successor thereto.
1.1.75 “Post-Reimbursement Approval Channel” means: (a) any customer for the Products in the Territory that treats patients with Major Depressive Disorder primarily on a reimbursed basis; and (b) any customers of Distributor for the Products obtained prior to receipt of the 1st Reimbursement Approval, whether the 1st Reimbursement Approval is obtained by Company or Distributor.
1.1.76 “Pre-Reimbursement Approval Channel” means any customer for the Products in the Territory during the period prior to receipt of the 1st Reimbursement Approval, whether the 1st Reimbursement Approval is obtained by Company or Distributor.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
6.
1.1.77 “Product” means: (a) the NeuroStar Product, Software, SenStar Connect, SenStar Treatment Links, NeuroStar Treatment Packs and those other items set forth in Schedules A and O attached to this Agreement, modified as necessary in order for the foregoing to receive the Regulatory Approvals; (b) all updates and improvements to any the foregoing that are marketed or sold by Company or its distributors in any country for Major Depressive Disorder indications; and (c) such additions, parts and accessories thereto as Company and Distributor mutually agree from time to time.
1.1.78 “Product Approval” means the approval by MHLW of a registration of the Product that allows for the importation, marketing, promotion, distribution and sale of the Product in the Territory as medical devices for the treatment of Major Depressive Disorder indications that are substantially similar to the Major Depressive Disorder indications for which the Products are approved in the United States with substantially the same conditions as approved in the United States.
1.1.79 “Product Catalog” means Company’s catalog of Products available for sale dated as of 2013, as modified by Company from time to time.
1.1.80 “Recall” has the meaning set forth in Section 6.8.
1.1.81 “Receiving Party” has the meaning set forth in Section 8.1.
1.1.82 “Redistributable Code” shall mean all third party software that is licensed to Company for redistribution with the Software.
1.1.83 “Regulatory Approvals” means (a) the Product Approval, (b) the Reimbursement Approval and (c) MAH/DMAH Approval.
1.1.84 “Regulatory Authorities” means the MHLW, PMDA and any other governmental body that has legal authority to regulate the manufacture, distribution or sale of medical devices in the Territory.
1.1.85 “Reimbursement Approval” means a determination by MHLW of the Reimbursement Rate.
1.1.86 “Reimbursement Approval Deadline” means the second anniversary of the date on which MHLW grants the first Product Approval.
1.1.87 “Reimbursement Rate” means the amount set by the MI-ILW from time to time that MHLW will reimburse hospitals and medical clinics for use of transcranial magnetic stimulation devices to treat patients suffering from those Major Depressive Disorder indications for which Company has obtained Product Approval.
1.1.88 “Return Policy” has the meaning set forth in Section 3.10.
1.1.89 “Rolling Termination Right” has the meaning set forth in Section 12.11.
1.1.90 “Rules” has the meaning set forth in Section 14.4.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
7.
1.1.91 “Sales Representatives” has the meaning set forth in Section 2.3.2.
1.1.92 “SenStar Connect” means Item Number ▇▇-▇▇▇▇▇-▇▇▇ as listed in the Product Catalog, a multiple-use consumable integrated flexible circuit that (a) must be attached to the treatment coil prior to MT or treatment to facilitate contact sensing and magnetic field detection and to decrease the magnetic field at the scalp surface to enhance tolerability during treatment and (b) is used in conjunction with a hygiene barrier.
1.1.93 “SenStar Treatment Link” means the current version of SenStar Treatment Links, Item Number ▇▇-▇▇▇▇▇-▇▇▇ as listed in the Product Catalog.
1.1.94 “Software” means the software programs, tools and data, whether in source or object code format, embedded or incorporated in the System or used in conjunction with the operation of the Products, including, without limitation, TrakStar Software, MT Assist, and Redistributable Code incorporated into or delivered with such software.
1.1.95 “Software Documentation” means [*], Rev B: Controlled Release of NeuroStar 2.3.1 System Software and [*], Rev C : Controlled Release of TrakStar 2.3.1 Software, as updated by Company from time to time.
1.1.96 “Specifications” means (a) prior to the Product Approval, the specifications for the Products set forth in Schedule M; and (b) after the Product Approval, the specifications for the Products as approved by MHLW from to time and the specifications for the Products set forth in Schedule M as amended by Company from time to time including in connection with changes to the Products.
1.1.97 [*]
1.1.98 “Steering Committee” has the meaning set forth in Section 2.5.1.
1.1.99 “Subsequent Year” means each full Fiscal Year immediately following the Baseline Year.
1.1.100 “System” means the Products, the Software, and single use items and other accessories sold by Company for use with the Products operating together as an integrated system or tool, including all successors, extensions, new models and upgrades thereto.
1.1.101 “Taxes” has the meaning set forth in Section 14.12.
1.1.102 “Technical Support Company” means [*] and such companies as Distributor may designate in writing to Company from time to time that will assist Distributor in the Territory with the delivery, installation and maintenance of Products and Software and provide technical support and such other assistance to end-user customers as requested by Distributor.
1.1.103 “Term” has the meaning set forth in Section 12.1.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
8.
1.1.104 “Territory” means Japan.
1.1.105 “Third Party Claim” has the meaning set forth in Section 10.1.1.
1.1.106 “TrakStar Software” means the Company’s practice data management system software including, if any, new versions, updates and upgrades thereto.
1.1.107 “TrakStar Computer” means a stand-alone personal computer that meets the specifications set forth on Schedule N, as such specifications may be updated by Company from time to time.
1.1.108 “Transition Effective Date” means the first business day that is [*] days before the expiration or termination of this Agreement.
1.1.109 “True-Up Payment” has the meaning set forth in Section 3.4.5.
1.1.110 “Vorpal” means Vorpal Technologies K.K.
1.1.111 “Warranty Period” has the meaning set forth in Section 9.3.1.
1.1.112 “Withholding Party” has the meaning set forth in Section 14.12.
1.1.113 “Year 2” means the first full Fiscal Year immediately following the first Subsequent Year.
2. Distributorship Terms.
2.1 Appointment and Acceptance.
2.1.1 Subject to the terms and conditions of this Agreement, Company hereby appoints Distributor as Company’s sole and exclusive distributor of Products and provider of NSTS in the Pre-Reimbursement Approval Channel and Post-Reimbursement Approval Channel, and Distributor accepts such appointment. During the Term, except as set forth in Section 2.1, Company shall (a) neither distribute any Products or provide any NSTS in the Pre-Reimbursement Approval Channel or the Post-Reimbursement Approval Channel nor appoint another distributor for the Products or NSTS in the Pre-Reimbursement Approval Channel or the Post-Reimbursement Approval Channel and (b) not sell Products to any third party that it knows or reasonably should know intends to resell the Products or provide NSTS in the Pre-Reimbursement Approval Channel or the Post-Reimbursement Approval Channel. Without limiting the generality of the foregoing, Company shall not solicit sales of Products or NSTS or promote the sale of Products or NSTS in the Territory except as specifically permitted by Sections 2.1.3 and 2.1.5. If Company receives an inquiry, purchase orders or other orders from a third party for delivery or sale of Products or provision of NSTS in the Pre-Reimbursement Approval Channel or Post-Reimbursement Approval Channel, Company shall promptly notify Distributor and refuse to fill any such inquiry, purchase order or other order unless Company is expressly permitted to do so by Sections 2.1.3 or 2.1.5.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
9.
2.1.2 Distributor specifically acknowledges that if, despite Company’s use of commercially reasonable efforts to prevent the unauthorized sale or resale of Products or NSTS in the Territory, Company is unable to stop or prevent such unauthorized sales or resale of Products or NSTS in the Territory in the Pre-Reimbursement Approval Channel or the Post-Reimbursement Approval Channel, then such unauthorized sale or resales of Products or NSTS in the Territory in the Pre-Reimbursement Approval Channel or the Post-Reimbursement Approval Channel shall not constitute a material breach of the terms of this Agreement, provided, however, that the Minimum Purchase Requirement shall be reduced by the amount of any such unauthorized sales or resale in the Post-Reimbursement Approval Channel.
2.1.3 Prior to receipt of the 1st Reimbursement Approval (whether obtained by Company or Distributor), Company shall have the right to sell Products and NSTS outside the Pre-Reimbursement Approval Channel in the Territory only for indications approved by the MHLW. Prior to receipt of the 1st Reimbursement Approval (whether obtained by Company or Distributor), Company shall have the right to sell Products and NSTS inside the Pre-Reimbursement Approval Channel in the Territory directly to: (a) [*]; provided that Company: (i) obtains [*] written agreement to comply with such use restriction and to not, as a normal course of business, distribute, resell, lease or otherwise transfer the Products to any third party; and (ii) uses commercially reasonable efforts to cause [*] compliance with such agreement; and (b) no more than one (1) customer (plus its Affiliates) other than [*], provided; that: (i) such customer must purchase the Products solely for use at medical clinics owned and operated by the customer or its Affiliates and not for resale; (ii) Company obtains the written agreement of the customer to not, as a normal course of business, distribute, resell, lease or otherwise transfer the Products to any third party; and (iii) Company uses commercially reasonable efforts to cause such customer to comply with such agreement.
2.1.4 Effective upon receipt of the 1st Reimbursement Approval (whether obtained by Company or Distributor), Company shall not, directly or indirectly, transfer, sell, or otherwise distribute itself or through any third party, any Products in the Pre-Reimbursement Approval Channel and the Post-Reimbursement Approval Channel except as set forth in Section 2.1.5.
2.1.5 After receipt of the 1st Reimbursement Approval (whether obtained by Company or Distributor), Company shall have the right to sell Products and NSTS (i) outside the Post-Reimbursement Approval Channel in the Territory for indications approved by the MHLW and (ii) in the Post-Reimbursement Approval Channel in the Territory directly to [*] or to [*] through [*] or a replacement distributor, solely for use at medical clinics owned and operated by [*], provided that Company: (a) obtains [*] and [*] written agreement to comply with such use restriction and to not, as a normal course of business, distribute, resell, lease or otherwise transfer the Products to any third party; and (b) uses commercially reasonable efforts to cause [*] and [*] compliance with such agreement. If [*] terminates its business involving use of the Products, Company may replace [*] with a customer (and its Affiliates) outside the Post-Reimbursement Channel in the Territory; provided that Company: (i) obtains such customer’s written agreement to be bound by and comply with the same restrictions as applicable to [*] and to not, as a normal course of business, distribute, resell, lease or otherwise transfer the Products to any third party; and (ii) uses commercially reasonable efforts to cause such customer’s compliance with such
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
10.
agreement. If Company has sold Products to one additional customer pursuant to clause (b) of Section 2.1.3, then upon receipt of the 1st Reimbursement Approval (whether obtained by Company or Distributor) Distributor shall have the option, exercisable by sending written notice to Company within [*] after Company confirms to Distributor in writing that such customer desires to use Products to treat patients on a reimbursed basis, to sell Products and NSTS to such customer in the Post-Reimbursement Approval Channel. If Distributor does not exercise such option, then Company shall have the right to sell Products and NSTS to such customer in the Post-Reimbursement Approval Channel, provided that: (i) such customer must purchase the Products solely for use at medical clinics owned and operated by the customer or its Affiliates and not for resale; (ii) Company obtains the written agreement of the customer to not, as a normal course of business, distribute, resell, lease or otherwise transfer the Products to any third party; and (iii) Company uses commercially reasonable efforts to cause such customer to comply with such agreement.
2.1.6 For the avoidance of doubt, nothing in this Agreement limits Company in relation to a [*] Device in the Territory.
2.2 No Activities Outside the Territory. Distributor shall not solicit sales of Products or promote the sale of Products outside the Territory. In the event Distributor receives an inquiry, purchase orders or other orders from a third party for delivery or sale of Products outside of the Territory, Distributor shall promptly notify Company and refuse to fill any such inquiry, purchase order or other order.
2.3 Sub-Distributors.
2.3.1 Distributor shall have the right to appoint its Affiliates as sub-distributors; provided that Distributor shall cause such Affiliates to comply with Distributor’s obligations under this Agreement and be liable to Company for any failures of such Affiliates to so comply.
2.3.2 Except as otherwise set forth in Section 2.3.1, Distributor shall not appoint any sub-distributor of Products in the Territory without the prior written consent of Company, which shall not be unreasonably withheld or conditioned; provided, however, Distributor may sell Products in the Territory to end-users in the Territory through Distributor’s sales representatives comprising part of its standard sales channels in the normal course of business (“Sales Representatives”), and such Sales Representatives shall not be deemed sub-distributors mentioned in this provision; provided, further, that: (a) Distributor shall cause each such Sale Representative to comply with Distributor’s obligations under this Agreement that are applicable to such Sales Representative’s activities; and (b) Distributor shall be liable to Company for any failure of the Sales Representative to comply with Distributor’s obligations under this Agreement that are applicable to such Sales Representative’s activities. For the avoidance of doubt, agents, distributors affiliated with hospitals, clinics and other end-users and unaffiliated distribution, logistics and similar companies designated by hospitals, clinics and other end-users for the purchase of Products shall not be deemed to be sub-distributors or Sales Representatives for purposes of this Agreement.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
11.
2.4 Independent Contractor Relationship. The relationship of Company and Distributor is that of independent contractors. This Agreement sets forth the duties and responsibilities of the Parties with respect to each other in furtherance of the purpose of this Agreement. This Agreement does not, however, give either Party the power to direct or control the day-to-day activities of the other. This Agreement further does not create or imply any relations between the Parties as partners, joint venturers, or co-owners. Neither Distributor nor its agents and employees are the representatives of Company for any purpose, and they shall have no power or authority as agent, employee or in any other capacity to represent, act for, bind, or otherwise create or assume any obligation on behalf of Company. Neither Company nor its agents, including the DMAH, and employees are the representatives of Distributor for any purpose, and they shall have no power or authority as agent, employee or in any other capacity to represent, act for, bind, or otherwise create or assume any obligation on behalf of Distributor. All financial obligations associated with Distributor’s business are the sole responsibility of Distributor. All financial obligations associated with Company’s business are the sole responsibility of Company. All sales and other agreements between Distributor and its customers are Distributor’s exclusive responsibility and do not affect Distributor’s obligations under this Agreement.
2.5 Steering Committee.
2.5.1 Establishment of Steering Committee. As soon as practicable after the Effective Date, the Parties shall establish a committee to facilitate the distribution of the Products in the Pre-Reimbursement Approval Channel and Post-Reimbursement Approval Channel in the Territory under this Agreement (the “Steering Committee”) in accordance with this Section 2.5.
2.5.2 Composition of the Steering Committee. The Steering Committee shall be comprised of one (1) representative designated by each of the Parties. Each representative shall be a senior executive of the designating Party. Each Party shall appoint its respective initial representative to the Steering Committee within [*] after the Effective Date, and may from time to time substitute its representative, in its sole discretion, effective upon notice to the other Party of such change. Additional representatives or consultants may from time to time be invited to attend Steering Committee meetings, subject to such representatives’ and consultants’ written agreement to comply with the requirements of Section 8 and the agreement of each Party. Each Party shall bear its own expenses relating to attendance at such meetings by its representatives and consultants.
2.5.3 Meetings. The Steering Committee shall meet in accordance with a schedule established by mutual written agreement of the Parties, but no less frequently than once per calendar year or as frequently as needed to discharge its responsibilities under this Agreement. The Steering Committee may meet by means of teleconference, videoconference or other similar communications equipment. In-person meetings shall alternate between a Company facility in the United States and a Distributor facility in Japan.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
12.
2.5.4 Matters for Steering Committee Discussion or Decision. The Steering Committee shall serve as a forum for discussion of development, regulatory, commercial and related matters concerning the Products in the Pre-Reimbursement Approval Channel and Post-Reimbursement Approval Channel in the Territory and have the following powers:
(a) approve the New Sales Forecast in accordance with and subject to Section 3.4.2;
(b) approve the transfer prices for the NeuroStar Starter Packages and other Products after the Fixed Transfer Price Period in accordance with and subject to Section 3.5.6;
(c) be presented with and discuss ideas and plans for updates and improvements to, and new versions of, the Products and Software for use in the Pre-Reimbursement Approval Channel and Post-Reimbursement Approval Channel; and
(d) be presented with and discuss information concerning competitive intelligence, market development and best practices concerning the marketing, promotion, distribution, sale and support of the Products and Software for use in the Pre-Reimbursement Approval Channel and Post-Reimbursement Approval Channel.
2.5.5 Decision-Making. With respect to the matters set forth in Sections 2.5.4(c) and 2.5.4(d), the Steering Committee has no decision making power as these matters are discussion only. With respect to the matters set forth in Sections 2.5.4(a) and 2.5.4(b), the Steering Committee shall use reasonable best efforts to reach unanimous agreement on a proposed decision with each Party (regardless of the number of attendees from the Party at a given meeting) having only one (1) vote. If the Steering Committee is unable to reach unanimous agreement on the matters set forth in Sections 2.5.4(a) or 2.5.4(b) within [*] days prior to the rules set forth in Sections 2.5.4(a) or 2.5.4(b), as applicable, controlling the decision, then a Party may by written notice to the other Party escalate the relevant decision to a senior executive appointed by each of the Parties. If the senior executives of both Parties are unable to reach agreement on the relevant decision within such [*] day period, then (a) for matters set forth in Section 2.5.4(a), Section 3.4.2 will control, and (b) for matters set forth in Section 2.5.4(b), Section 3.5.6 will control.
3. Prices and Terms.
3.1 Initial Payment; Milestone Payments.
3.1.1 Distributor shall pay Company a non-refundable initial payment of Seven Hundred and Fifty Thousand Dollars ($750,000) (the “Initial Payment”), such payment to be made by the end of the month following the month in which the Effective Date falls and the Distributor receives an invoice for the Initial Payment.
3.1.2 Distributor shall pay Company a non-refundable milestone payment of Two Million Dollars ($2,000,000) (the “Milestone Payment 1”) by the end of the month following the month in which Company obtains the first Product Approval for the Product in the Territory, provides written notice thereof to Distributor and Distributor receives an invoice for Milestone Payment 1.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
13.
3.1.3 Distributor shall pay Company a second non-refundable (other than a potential true-up payment required by Section 3.1.7) milestone payment based on the Reimbursement Rate in accordance with the following formula (“Milestone Payment 2”):
[*]
3.1.4 If the 1st Reimbursement Approval is not obtained by the Reimbursement Approval Deadline and Distributor makes the Distributor 1st MHLW Lead Election under Section 4.1.2, then Milestone Payment 2 shall be deemed fully earned and the Reimbursement Rate used in calculating Milestone Payment 2 shall be [*].
3.1.5 If Company obtains the 1st Reimbursement Approval by the Reimbursement Approval Deadline and the Reimbursement Rate is less than [*] and neither Party terminates this Agreement pursuant to Section 12.7.1, then Distributor shall pay Initial Milestone Payment 2 using the Reimbursement Rate in the 1st Reimbursement Approval. Milestone Payment 2 shall then be finally calculated as follows:
(a) If the Reimbursement Rate set in the 1st Reimbursement Revision Approval during the Company MHLW Lead Period is less than [*] and Distributor makes the Distributor 2nd MHLW Lead Election under Section 4.1.4, then Milestone Payment 2 shall be calculated using [*] as the Reimbursement Rate.
(b) if the Reimbursement Rate set in the 1st Reimbursement Revision Approval during the Company MHLW Lead Period is less than [*] and Distributor does not make the Distributor 2nd MHLW Lead Election under Section 4.1.4, then regardless of whether this Agreement is terminated pursuant to Section 12.8.1, Milestone Payment 2 shall be calculated using the Reimbursement Rate set in the 1st Reimbursement Revision Approval.
(c) If the Reimbursement Rate set in the 1st Reimbursement Revision Approval during the Company MHLW Lead Period is at least [*], then Milestone Payment 2 shall be calculated using the Reimbursement Rate set in the 1st Reimbursement Revision Approval.
3.1.6 If the Company obtains the 1st Reimbursement Approval by the Reimbursement Approval Deadline and the Reimbursement Rate is at least [*], then Distributor shall pay Initial Milestone Payment 2 using the Reimbursement Rate in the 1st Reimbursement Approval. Milestone Payment 2 shall then be finally calculated as follows:
(a) If the Reimbursement Rate set in the 1st Reimbursement Revision Approval during the Company MHLW Lead Period is less than [*] and either Party elects to terminate the Agreement pursuant to Section 12.7.2, then Milestone Payment 2 shall be calculated using the Reimbursement Rate set in the 1st Reimbursement Revision Approval.
(b) If the Reimbursement Rate set in the 1st Reimbursement Revision Approval during the Company MHLW Lead Period is less than [*] and neither Party elects to terminate the Agreement pursuant to Section 12.7.2, then Milestone Payment 2 shall be calculated using the Reimbursement Rate set in the 1st Reimbursement Revision Approval.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
14.
(c) If the Reimbursement Rate set in the 1st Reimbursement Revision Approval during the Company MHLW Lead Period is [*] and Distributor makes the Distributor 2nd MHLW Lead Election under Section 4.1.4, then Milestone Payment 2 shall be calculated using [*] as the Reimbursement Rate.
(d) If the Reimbursement Rate set in the 1st Reimbursement Revision Approval during the Company MHLW Lead Period is less than [*] and Distributor does not make the Distributor 2nd MHLW Lead Election under Section 4.1.4 and the Agreement is terminated pursuant to Section 12.8.1, then Milestone Payment 2 shall be calculated using the Reimbursement Rate set in the 1st Reimbursement Revision Approval.
(e) If the Reimbursement Rate set in the 1st Reimbursement Revision Approval during the Company MHLW Lead Period is at least [*], then Milestone Payment 2 shall be calculated using the Reimbursement Rate set in the 1st Reimbursement Revision Approval.
3.1.7 Once Milestone Payment 2 is finally calculated pursuant to Sections 3.1.5 or 3.1.6, one of the Parties shall make a payment to the other as follows: (a) if Milestone Payment 2 (as finally calculated) is more than Initial Milestone Payment 2, Distributor shall pay Company an amount equal to the difference of Milestone Payment 2 (as finally calculated) minus Initial Milestone Payment 2; or (b) if Milestone Payment 2 (as finally calculated) is less than Initial Milestone Payment 2, Company shall refund Distributor an amount equal to the difference of Initial Milestone Payment 2 minus Milestone Payment 2 (as finally calculated).
3.1.8 Distributor shall pay Company Initial Milestone Payment 2 and Milestone Payment 2 in US Dollars at a fixed exchange rate of [*] to One Dollar ($1), by the end of the month following the month in which Distributor receives an invoice from Company setting forth a correct calculation of the amounts due to Company. For amounts that Company must refund to Distributor pursuant to Section 3.1.7, if any, Company shall provide Distributor with a credit in the amount of the refund and Distributor may apply such credit at its discretion against other amounts owed to Company pursuant to this Agreement. If the credit is not exhausted by the effective date of termination of this Agreement, Company shall pay the remaining balance of the credit in US Dollars by wire transfer in immediately available funds to such bank account as designated by Distributor no later than [*] after the date this Agreement terminates.
3.2 Delivery Lead Time; Order Forecasts.
3.2.1 Company will notify Distributor of the delivery date(s) for Products within [*] after receipt of Distributor’s purchase order placed pursuant to Sections 3.2.3 or 3.3.2; provided that such delivery date(s) will be no longer than [*] from the date of Distributor’s purchase order.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
15.
3.2.2 Distributor shall, on a monthly basis commencing on the [*] of the month following the month in which the Effective Date falls and thereafter within the initial [*] of each succeeding month, provide Company with Distributor’s good faith [*] forecast of Distributor’s monthly requirements for the Products, including the NeuroStar Starter Package (each, an “Order Forecast”). Distributor’s first Order Forecast may not include any NeuroStar Starter Package for delivery in months [*] of such Order Forecast or have more than [*] of NeuroStar Starter Package in months [*] of such Order Forecast except with Company’s consent in its sole discretion. Distributor may not adjust months [*] of any subsequent Order Forecast except with the consent of Company in its sole discretion. Until Distributor issues a purchase order, Distributor may adjust the forecast for months five and six of any Order Forecast in succeeding Order Forecasts (i.e. when months [*] on an Order Forecast become months [*] on the next Order Forecast) to an amount between [*] of the NeuroStar Starter Package per month. If Distributor adjusts the forecast for NeuroStar Starter Packages in month [*] of an Order Forecast to an amount greater than [*] in month [*] on the next Order Forecast, then Company will use commercially reasonable efforts to respond within [*] as to the extent to which Company will be able to fulfill such a request by Distributor. If Company notifies Distributor that Company can fulfill such request, in whole or in part, then the Order Forecast for the applicable month will be increased above [*] by the additional number of units that Company agrees to deliver in such month. If Company cannot fulfill such request by Distributor, then the Order Forecast will be [*] of the NeuroStar Starter Package for the applicable month. Distributor agrees to use commercially reasonable efforts to make each Order Forecast as accurate as possible.
3.2.3 Promptly after the Effective Date Company and Distributor shall discuss in good faith the initial quantity of Products other than the NeuroStar Starter Package that Distributor will purchase from Company and the timing of when Distributor will place its initial purchase order for such Products. Distributor will place its initial purchase order for such quantities of such Products and at such time as agreed by Company and Distributor.
3.3 Purchase Orders.
3.3.1 During the Term, Distributor shall order Products from Company by submitting monthly written purchase orders identifying: Products ordered by catalog number and quantity and requested delivery date(s); provided that: (a) NeuroStar Starter Packages shall be subject to a minimum individual order of [*]; (h) SenStar Treatment Links shall be subject to a minimum individual order of [*], packaged in the 50 pack form; (c) Treatment Session Treatment Packs (200 Packs per box) shall be subject to a minimum individual order of [*]; and (d) spare parts can only be ordered on a quarterly basis in quantities to replenish (or increase) Distributor’s normal inventory of spare parts (which normal inventory until the second anniversary of the date of Product Approval will be equal to or greater than Company’s recommended spare parts inventory level and thereafter will be determined by Distributor with reference to its experience servicing the Products in the Territory for the installed base of NeuroStar Starter Packages in the Territory), except that until the second anniversary of the date of Product Approval, if there is a stock out of a particular spare part or an unexpected need for a spare part not normally carried in inventory, Company will accept ad hoc purchase orders therefor. The NeuroStar Product cannot be ordered separate from a NeuroStar Starter Package.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
16.
3.3.2 At the same time that Distributor sends each Order Forecast to the Company (and in any event no later than the date by which Distributor must send the applicable Order Forecast to the Company), Distributor shall issue purchase orders to Company for the first [*] of requirements for each of the NeuroStar Starter Packages shown on such Order Forecast and, after issuing the initial purchase order pursuant to Section 3.2.3, for such other Products as Distributor desires to order. Company may elect to treat months one through four of each Order Forecast as binding purchase orders for NeuroStar Starter Packages if Distributor fails to issue the required purchase order on a timely basis.
3.3.3 An purchase orders for Products are subject to acceptance by Company; provided that Company must accept a purchase order if the order complies with this Agreement including the limitations and requirements of Sections 3.2 and 3.3.1. If Company rejects a purchase order that it is permitted to reject, Company shall notify Distributor within [*] after receiving the purchase order and state the reason(s) for rejecting the purchase order. If Company does not notify Distributor of the rejection of the purchase order and state the reason(s) for rejecting the purchase order within the [*], the purchase order shall be deemed accepted. If Company does not accept a purchase order for Products, in whole or part, that otherwise complies with this Agreement including the limitations and requirements of Sections 3.2 and 3.3.1, then Distributor shall receive a credit against its then-current Minimum Purchase Requirement for the amount of Products that Distributor would have paid Company if Company had accepted the rejected portion of such purchase order.
3.3.4 Purchase orders placed by Distributor and accepted by Company and the delivery of Products by Company pursuant to such purchase orders shall not be canceled or rescheduled unless mutually agreed upon by both Parties.
3.3.5 If Company does not deliver NeuroStar Starter Packages or other Products by the dates set forth in purchase orders that Company has accepted pursuant to this Section 3.3, other than by (a) Distributor not providing all shipping information required by Company including approved carriers, departure port, vessel and sail date within forty (45) days prior to shipment or (b) damage, loss or other casualty while in transit from Company to the delivery point in the Territory, then, in addition to any other rights or remedies that Distributor may have, Company shall provide Distributor with a credit to be applied toward Distributor’s future purchase of Products for each day that delivery of the Products is delayed over fifteen (15) days from the delivery date that shall be calculated by multiplying the purchase price of the Products for which delivery is delayed times 6% and dividing the resulting amount by 360.
3.4 Minimum Purchase Requirement.
3.4.1 After the 1st Qualifying Approval is obtained and for the remainder of the Term, Distributor shall be required to purchase a minimum annual Dollar value of the Products from Company based on the sales forecasts for the Products in the Territory (“Minimum Purchase Requirement”) or pay Company the True-Up Payment (as set forth in Section 3.4.5) unless this Agreement is terminated as set forth in Section 12, in which case Distributor’s Minimum Purchase Requirement and True-Up Payment obligations shall be as set forth in Section 12, as applicable.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
17.
3.4.2 The sales forecast to be used to determine the Minimum Purchase Requirement for the period from the date on which [*] (the “Initial Period”) is set forth in Schedule B (the “Initial Sales Forecast”). No later than [*] prior to the end of each Fiscal Year (starting with the last Fiscal Year of the Initial Period), the Steering Committee shall meet and discuss in good faith the sales forecast of Products in the Territory for the next Fiscal Year of Distributor during the Term (each, “New Sales Forecast”). If the Steering Committee and senior executive escalation process set forth in Section 2.5.5 does not result in an agreed New Sales Forecast for any such Fiscal Year by [*], then the New Sales Forecast for such Fiscal Year shall equal [*] However, if the Company commences the sale or promotion of [*] Devices in the Territory, then [*] For clarity, each New Sales Forecast reflects the Dollar value of the forecasted amount of purchases of the Products by Distributor from Company during the relevant Fiscal Year.
3.4.3 The Minimum Purchase Requirement for the Initial Period shall be [*]; provided, however, that all purchases of Products after the Product Approval shall count toward satisfying the Minimum Purchase Requirement for the Initial Period.
3.4.4 For each [*], the Minimum Purchase Requirement will be adjusted in accordance with the following:
(a) If Distributor’s purchases of Products from Company [*] the Initial Sales Forecast for the Initial Period or the New Sales Forecast for any subsequent Fiscal Year, then the Minimum Purchase Requirement [*] for the next succeeding Fiscal Year shall be [*] (which by way of example means that if Distributor [*] the Initial Sales Forecast for the Initial Period, then the Minimum Purchase Requirement for the next Fiscal Year shall be [*];
(b) If Distributor’s purchases of Products from Company [*] the Initial Sales Forecast for the Initial Period or the New Sales Forecast for any subsequent Fiscal Year, then the Minimum Purchase Requirement [*] for the next succeeding Fiscal Year shall be [*];
(c) Except as set forth in Section 34.4(d), the Minimum Purchase Requirement [*] shall [*];
(d) Notwithstanding Sections 3.4.4(a), 3.4.4(b) and 3.4.4(c), if Distributor commences sales of [*] Devices in the Territory, Company has not sold or promoted [*] Devices in the Territory prior thereto and Distributor’s Dollar value of purchases of Products during any Subsequent Year are [*] of the [*] Minimum, then the Minimum Purchase Requirement for that Subsequent Year shall [*]; and
(e) The Minimum Purchase Requirement for [*] shall not be more than [*]
3.4.5 Distributor shall satisfy its Minimum Purchase Requirement obligations for [*] by either: (a) purchasing the Dollar value of Products from Company equal to the Minimum Purchase Requirement; or (b) paying Company an amount equal to [*] (each, a “True-Up Payment”). Distributor shall pay Company each True-Up Payment within [*] and receipt of an invoice from Company. If Distributor satisfies its Minimum Purchase Requirement
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
18.
obligations for any period under clause (b) Distributor shall also have the option to terminate this Agreement on [*] prior written notice to Company and [*]. For the avoidance of doubt, payment of a True-Up Payment shall not be considered as achieving [*] for purposes of calculating Minimum Purchase Requirement [*].
3.5 Transfer Prices.
3.5.1 The initial transfer price for each NeuroStar Starter Package shall be [*] (the “Initial Transfer Price”) [*] (the “Fixed Transfer Price Period”).
3.5.2 The transfer price of the SenStar Treatment Link will be Sixty Dollars ($60) per one SenStar Treatment Link unit until Company [*] in accordance with Distributor’s reasonable requirements and Company [*]. If Company does not obtain a Reimbursement Approval in the Territory that [*] within [*], then Company and Distributor shall discuss an adjustment in the transfer price of SenStar Treatment Links in good faith. Upon agreeing to an adjustment in the transfer price of SenStar Treatment Links, the Parties shall amend Schedule J to set forth the new agreed upon transfer price for SenStar Treatment Links.
3.5.3 Until Reimbursement Approval that includes [*] is obtained in the Territory, the transfer price for orders of [*] will be [*] during [*] and thereafter will be [*].
3.5.4 Distributor must separately order NeuroStar Treatment Packs as needed from Company.
3.5.5 The transfer price for orders of NSTS placed after the date of the Reimbursement Approval will be determined as set forth in Schedule J for the Fixed Transfer Price Period; provided, however, that if during the Fixed Transfer Price Period the Reimbursement Rate changes, then the NSTS transfer price for orders placed after the Reimbursement Rate change will be determined in accordance with Schedule J based on the new Reimbursement Rate.
3.5.6 The transfer price for orders of all Products set forth on Schedule O but not set pursuant to Sections 3.5.1 through 3.5.5 will be as set forth on Schedule O and fixed for the Fixed Transfer Price Period.
3.5.7 [*] prior to the end of the Fixed Transfer Price Period, Company and Distributor shall, through the Steering Committee, meet to discuss in good faith changes to the transfer prices for the NeuroStar Starter Package and all other Products based on all relevant factors. All new transfer prices for such Products agreed to by Company and Distributor shall apply for a period of [*]. [*] the Steering Committee shall meet and discuss in good faith changes to such transfer prices based on the factors set forth in the preceding sentence. If the Steering Committee and the escalation process does not result in mutually agreed revised transfer prices as contemplated by this Section by [*] prior to the end of the Fixed Transfer Price Period or then-current [*], then the transfer prices shall be increased by [*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
19.
3.5.8 Company shall notify Distributor when Company commences development of a Product that incorporates major improvements in functionality over the then-current version of the Product and provide periodic status reports regarding the development of such Product to Distributor. Prior to any sale of any Product in the Territory that incorporates major improvements in functionality over the then current version of the Product, the Parties shall meet and discuss in good faith an adjustment to the transfer price for such Product.
3.6 Resale Pricing. Distributor shall be free to establish its own resale pricing for Products that it distributes in the Territory.
3.7 Shipping and Delivery Terms.
3.7.1 Company shall ship all Products ordered by Distributor pursuant to this Agreement DDP (Incoterms 2010) cleared through customs to Distributor’s or the Technical Support Company’s facility in Japan as notified by Distributor to Company in writing; provided that the following exceptions to DDP shall apply: (a) Company will use the carrier or carriers approved by Distributor unless such carrier(s) indicate(s) that they cannot deliver the Products at least [*] prior to the applicable delivery date in which case Company may use an appropriate alternative carrier that is able to timely deliver the Products; (b) Distributor will reimburse Company for the cost of shipping from Company’s warehouse to the point of delivery in the Territory, associated freight insurance and import duties and tariffs, which amounts Company will invoice to Distributor on a pass-through basis within [*] after the end of each month and accompanied by copies of invoices or receipts that reasonably document the costs of shipping, insurance and import duties and tariffs for which Company is seeking reimbursement from Distributor; and (c) Company’s liability to Distributor for damage, loss or other casualty to Products for the period from the shipment from Company’s warehouse to the delivery point in the Territory will be exclusively limited to prompt re-supply of the same number of Products so damaged, lost or subject to casualty. Company will package each such shipment in accordance with standard practices acceptable to mode of shipment chosen by Distributor unless Company is permitted pursuant to clause (a) of this Section 3.7.1 to use a different mode of shipping.
3.7.2 If there is any shortage in the quantity of Products delivered by Company and Distributor notifies Company within [*] after delivery, Company shall promptly deliver replacement Products in accordance with the terms of this Agreement at Company’s expense to make up such shortfall.
3.8 Payment. Unless otherwise specified in this Agreement and unless subject to a bona fide dispute, Distributor shall pay all amounts due and payable under this Agreement by the end of the month following the month in which the Products have been delivered in accordance with this Agreement and Distributor receives an invoice for such Products. Company reserves the right to withhold shipments of Products or to require payment in full prior to shipment of Products in the event any amounts due Company by Distributor are past due unless such amounts are subject to a bona fide dispute. All such amounts, and any other payment due pursuant to the terms of this Agreement, shall be paid by wire transfer in United States Dollars to the bank listed below (or such other wire transfer instructions or bank as Company may specify in writing from time to time) or by other means specified in writing and mutually agreed by both Parties to:
Pay to: [*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
20.
Routing and transit no.: [*]
SWIFT Code: [*]
For credit of: [*]
Final credit account no.: [*]
By order of: (Sender’s name)
All costs incurred in connection with any such wire transfer shall be the responsibility of Distributor. Amounts due under this Agreement shall be considered paid as of the day such funds are received by the aforementioned bank.
3.9 Late Payments. In addition to the other rights of Company under this Agreement, all amounts due and owing to Company under this Agreement, but not paid by Distributor on the due date thereof (excluding amounts subject to a bona fide dispute), shall bear interest (in U.S. Dollars) at the lower of the of [*] per annum or the maximum lawful interest rate permitted under applicable law. Such interest shall accrue on the balance of unpaid amounts from time to time outstanding from the date on which portions of such amounts become due and owing until payment thereof in full.
3.10 Return of Products. Distributor and Company shall follow the procedures set forth in Schedule F with respect to allegedly Defective Products.
3.11 Governing Document. This Agreement, together with its Schedules, shall supersede any additional, conflicting or supplemental terms used by Company or Distributor in the ordering, shipment and receiving of Products, including without limitation, any purchase orders or order acknowledgements other than ministerial items such as shipping address, delivery date and quantities.
3.12 [*] Credit. If Distributor commences the sale of [*] Devices in the Territory [*], then Company shall [*]
4. Regulatory Approvals; Distributor Approvals; Interactions with Regulatory Authorities.
4.1 Regulatory Approvals.
4.1.1 Company shall use commercially reasonable efforts to obtain and shall thereafter maintain all Regulatory Approvals including, in connection with the first Regulatory Approval, a use results survey period (exclusivity) of [*] and shall provide all information required to be submitted to the Regulatory Authorities or which the Regulatory Authorities request in connection with the Iryoukiki no Seizo Hanbai Go no Chousa oyobi Shiken no Jisshi no Kijun ni kansuru Shourei (Ministerial Ordinance on Standards for Post Market Surveillance and Testing of Medical Equipment); provided that Company shall not be required to (a) undertake any clinical trial in order to obtain the Regulatory Approvals including post-marketing studies required by MHLW as a condition to granting any of the Regulatory Approvals unless Distributor agrees to fully fund such post-marketing studies or (b) make changes to the form, fit or function of any of the Products, except as set forth in Section 6.1. Distributor shall reasonably cooperate with Company’s efforts to secure the Regulatory Approvals.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
21.
4.1.2 If, notwithstanding the exercise of its commercially reasonable efforts, Company does not obtain the 1st Reimbursement Approval by the Reimbursement Approval Deadline, then Distributor may elect to take over the process for obtaining the Reimbursement Approval with the assistance of Vorpal or such other third party(ies) as Distributor desires (the “Distributor 1st MHLW Lead Election”); provided that such election is made by written notice to Company within ninety (90) days after the Reimbursement Approval Deadline. If Distributor makes the Distributor 1st MHLW Lead Election, then Distributor shall use its commercially reasonable efforts during the Distributor 1st MHLW Lead Period to obtain the 1st Reimbursement Approval, subject to the termination rights set forth in Section 12.6.2.
4.1.3 If Company obtains the 1st Reimbursement Approval by the Reimbursement Approval Deadline and either (a) the Reimbursement Rate is [*] and neither Party terminates this Agreement pursuant to Section 12.7.1 or (b) the Reimbursement Rate is [*], then Company shall use its commercially reasonable efforts until the 1st Reimbursement Revision Approval is issued by MHLW to attempt [*].
4.1.4 If Company obtains the 1st Reimbursement Approval by the Reimbursement Approval Deadline and notwithstanding the exercise of commercially reasonable efforts, Company is not able [*] then Distributor may elect to take over the process for obtaining the Reimbursement Approval with the assistance of Vorpal or such other third party(ies) as Distributor desires (the “Distributor 2nd MHLW Lead Election”), provided that such election is made by written notice to Company within ninety (90) days after the date of the 1st Reimbursement Revision Approval. If Distributor makes the Distributor 2nd MHLW Lead Election, then Distributor shall use its commercially reasonable efforts during the Distributor 2nd MHLW Lead Period [*] subject to the termination rights set forth in Section 12.8.2.
4.2 Distributor Approvals. Distributor shall use commercially reasonable efforts to obtain and thereafter maintain all approvals, permits and licenses not within the definition of Regulatory Approvals required for import, marketing and sale of the Products in accordance with the Product Approval including a medical device retail and leasing license for the Product in the Territory (collectively, the “Distributor Approvals”).
4.3 Initial Designation of DMAH and Replacement. Company’s Agent shall be the initial Designated Marketing Authorization Holder in the Territory. If Company is unable to be hold any of the Regulatory Approvals or Company’s Agent is unable to be the Designated Marketing Authorization Holder in Japan or is unable to supply Distributor with Products as set forth in Section 6.3, Distributor shall have the right to become the MAH instead of Company and/or Company’s Agent for purposes of allowing Distributor to continue to market and sell Products in the Territory for business continuity purposes. In such event, Company shall cooperate with Distributor to effect such changes.
4.4 Interactions with Regulatory Authorities.
4.4.1 Subject to Distributor’s rights set forth in Sections 4.1 and 4.3, Company is responsible for all interactions with regulatory authorities in the Territory (including MHLW) which are conducted through the DMAH. Distributor shall reasonably assist Company in any
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
22.
regulatory request or action by providing customer or product information that is requested by regulatory authorities worldwide from Company or the DMAH and support any regulatory actions required in the Territory. Notwithstanding the foregoing, Distributor will lead all interactions with the MHLW concerning reimbursement during the Distributor 1st MHLW Lead Period and the Distributor 2nd MHLW Lead Period and Company shall reasonably assist Distributor.
4.4.2 Company and the DMAH are responsible for medical device reporting for complaints and/or adverse event reports, conducting such reporting to regulatory agencies in the Territory in compliance with applicable regulatory timelines and regulations, and for reporting complaints and/or adverse event reports to any other jurisdiction including, but not limited, to the United States. Company is also responsible for working with the DMAH on any issues that arise in clearing the Products through customs in the Territory. For the avoidance of doubt, Distributor shall have no such responsibility.
5. Distributor’s Duties.
5.1 Distributor’s Efforts. Upon Company’s receipt of the Regulatory Approvals, Distributor shall use its commercially reasonable efforts to diligently promote the sale of Products in the Territory, in compliance with Applicable Law. Distributor shall do nothing to detract from the good name of Company or the reputation of Products. Without limiting the generality of the foregoing, Distributor shall have the following obligations with respect to the advertising, promotion, marketing, distribution and sale of Products:
5.1.1 To undertake its marketing, promotional, distributional and selling activities for the Products in the Territory at its own risk and expense using a field sales force as soon as reasonably commercially practical after receipt of the Regulatory Approvals;
5.1.2 To install and maintain all Products sold to Distributor and its Affiliates in the Territory;
5.1.3 To negotiate in good faith the terms of a memorandum of good vigilance practice with the DMAH and to comply with the terms of that memorandum once agreed to;
5.1.4 To use commercially reasonable efforts to advertise and promote Products diligently in the Territory, in compliance with Applicable Laws as soon as commercially reasonable after receipt of the Regulatory Approvals;
5.1.5 To attend and assist at trade shows, physician meetings and other professional gatherings in the Territory, to the extent Distributor deems it appropriate to promote the sale of Products as soon as reasonably practical after receipt of the Regulatory Approvals. From time to time, Company may make specific reasonable requests of Distributor to attend or assist in international trade shows, physician meetings and other professional gatherings outside the Territory;
5.1.6 To maintain an adequate inventory of Products and spare parts to support the installed base of Products in the Territory;
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
23.
5.1.7 To work according to, and perform its responsibilities under, the Distributor Quality Plan;
5.1.8 To follow-up on sales leads from potential customers in the Territory referred to Distributor by Company;
5.1.9 Provide Company prior to the commencement of each Fiscal Year: (a) an annual financial forecast in the Territory that includes Distributor’s assumptions for the Territory; and (b) Distributor’s annual marketing plan that includes information concerning Competitive Product that Distributor is then aware of, trade shows and workshops to be held in the Territory, advertising and promotion for the Product in the Territory and product and marketing needs in the Territory; and
5.1.10 Within [*] after the end of each calendar quarter, provide Company with a report describing performance against the then-current sales forecast (i.e. Initial Sales Forecast or then-current New Sales Forecast) and marketing plan and a brief summary of the reasons for positive and negative variances from such sales forecast and plan with the format of such report being substantially in the form of Schedule G. The provision of such information to Company does not, in and of itself, provide Company with a right to terminate this Agreement that is independent from and/or in addition to Company’s express termination rights under this Agreement.
5.2 Clinical Studies.
5.2.1 Except as set forth in Section 5.2.2, Distributor shall not conduct or otherwise support any study of the Products in the Territory without the prior written consent of Company.
5.2.2 Distributor shall solely fund all post-marketing clinical studies in the Territory subsequently agreed to by Distributor and Company in writing but excluding all post-marketing studies in the Territory required by the MHLW in connection with obtaining the Regulatory Approvals. Distributor’s obligations under this Section 5.2.2 shall be limited to the direct expenses of clinical studies incurred by Company after the Effective Date and Distributor shall have no obligation with respect to any costs and expenses incurred prior to the Effective Date.
5.2.3 Distributor shall reasonably cooperate with Company concerning the implementation of clinical studies of the Products in the Territory described in Section 5.2.2 for the Major Depressive Disorder indications referred to therein.
5.3 TrakStar Computer. Distributor shall be responsible for obtaining and supplying to each purchaser of the NeuroStar Product a TrakStar Computer that is dedicated to use with the NeuroStar Product. Distributor may charge the customer whatever price it determines for the TrakStar Computer and, as between Distributor and Company, shall be solely responsible for all warranties and service with respect to the TrakStar Computer.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
24.
5.4 Company Audit Rights. Distributor shall ensure that an independent third party selected by Company and reasonably acceptable to Distributor and the Regulatory Authorities, to the extent permitted by Applicable Law, may, during regular business hours, after entering into a confidentiality agreement reasonably acceptable to Distributor and upon reasonable advance written notice, not more than once annually, (a) examine and inspect Distributor’s facilities or, subject to any third party confidentiality restrictions and other obligations, the facilities of any Affiliate or Sales Representatives involved in the promotion or sales of the Products in the Territory, and (b) subject to Applicable Law and any third party confidentiality restrictions and other obligations, inspect all data, documentation and work product relating to the activities performed by Distributor and Distributor’s Affiliates and Sales Representatives engaged in the promotion and sales of Products in the Territory solely for the purposes of determining Distributor’s compliance with the terms of this Agreement. If an audit discloses that Distributor is not in material compliance with the terms of this Agreement, then notwithstanding the previous sentence Company shall have the right to conduct a follow-up audit during the same year or thereafter to confirm that the deficiencies discovered during the initial audit have been corrected by Distributor and Distributor is in material compliance with the terms of this Agreement. This right of Company to audit and inspect the data and documentation of Distributor and Distributor’s Affiliates and Sales Representatives involved in the promotion and sales of the Products in the Territory may be exercised at any time during the Term upon reasonable notice (subject to each Party’s record retention policies then in effect), or such longer period as shall be required by Applicable Law.
5.5 Payments to Government Officials.
5.5.1 Distributor shall not directly or indirectly, for the purpose of obtaining approval, promoting, selling or distributing Products, offer, pay, or promise to pay, or provide any money, service, gift, or thing of value to any official, agent, employee or representative of a government or government agency such as a public hospital (hereafter collectively “Government Official”), and shall otherwise comply with the laws and regulations then in effect, if any, governing interactions with Government Officials, including but not limited to, the U.S. Foreign Corrupt Practices Act to the extent applicable to Distributor (hereafter, the “Anti-Corruption Laws”). Distributor further makes the following representations and warranties as of the Effective Date and covenants in connection with its activities related to this Agreement:
(a) Distributor and its Affiliates are solely responsible for complying, have to its best knowledge complied, and shall comply, with the Anti-Corruption Laws in connection with performing the obligations of Distributor set forth in this Agreement and have to the best of its and their knowledge not taken and shall not take or fail to take any action, which act or failure would subject Company to liability under Anti-Corruption Laws in connection with performing the obligations of Distributor set forth in this Agreement; and
(b) Neither Distributor nor any of its Affiliates has, to its or their best knowledge, offered, paid, given or loaned or promised to pay, give or loan, or will not offer, pay, give or loan or promise to pay, give or loan, directly or indirectly, money or any other thing of value to or for the benefit of any Government Official in connection with performing the obligations of Distributor set forth in this Agreement, for the purposes of corruptly: (i)
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
25.
influencing any act or decision of such Government Official in his official capacity; (ii) inducing such Government Official to do or omit to do any act in violation of his lawful duty; (iii) securing any improper advantage; or (iv) inducing such Government Official to use his influence with a government entity to affect or influence any act or decision of that Government Official, in each instance to direct business to Distributor or Company.
5.5.2 Distributor shall assist and cooperate fully with the efforts of Company to comply with the Anti-Corruption Laws. In particular, Distributor shall keep accurate books and records and Distributor shall immediately notify Company of any information that bribes or other improper payments are being requested, made or offered by Distributor or its Affiliates in connection with this Agreement. Upon request of Company, with Distributor’s prior written consent and to the extent applicable to Distributor, Distributor shall make those records which are necessary for Company to verify Distributor’s compliance with the Anti-Corruption Laws relating to this Agreement available to an auditor selected by Company. If such auditor notices any failure by Distributor to comply with the Anti-Corruption Laws, Distributor agrees that the auditor may disclose information relating to such Distributor’s failure to Company and, to the extent required by a legal demand by a competent court of law or government body, to third parties.
5.5.3 Distributor shall truthfully and accurately complete the distributor qualification form and anti-bribery certification which are attached to Schedule H and deliver such documents to Company promptly after the Effective Date and dated the Effective Date. On each anniversary of the Effective Date during the Term, or the next succeeding business day if any anniversary is not a business day, Distributor shall deliver to Company an updated anti-bribery certification using Company’s form of anti-bribery certification then required by Company’s anti-bribery and anti-corruption policy (currently document number ▇▇-▇▇▇▇▇-▇▇▇, Rev. A).
5.5.4 In no event shall Company be obligated to Distributor under or in connection with this Agreement to act or refrain from acting if Company believes that such act or omission would cause Company to be in violation of the Anti-Corruption Laws. In no event shall Company be liable to Distributor for any act or omission which Company believes is necessary to comply with the Applicable Law. In no event shall Distributor be obligated to Company under or in connection with this Agreement to act or refrain from acting if Distributor believes that such act or omission would cause Distributor to be in violation of the Anti-Corruption Laws. In no event shall Distributor be liable to Company for any act or omission which Distributor believes is necessary to comply with Applicable Law.
5.5.5 If Distributor or any of its Affiliates breaches any of the representations, warranties or covenants in this Section 5.5, and each of which is deemed to be material and continuously made throughout the Term, then, in addition to any other rights Company may have under this Agreement:
(a) Company may declare a forfeit of any unpaid amounts owing to Distributor and shall be entitled to repayment of any amounts paid or credited to Distributor, in each case, which are prohibited by the Anti-Corruption Laws; and
(b) Company may immediately terminate this Agreement upon written notice to Distributor.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
26.
5.6 Compliance with Code of Conduct for Interactions with Healthcare Providers. Distributor agrees to comply with the Japan Federation of Medical Devices Associations’: (a) Code of Ethics, as amended from time to time; and (b) Promotion Code of the Medical Devices Industry, as amended from time to time ((a) and (b), together, the “Code”), and to ensure that all marketing and sales interactions with customers will be conducted in compliance with the Code.
5.7 Sales and Clinical Training. Distributor shall be responsible for training its personnel who promote Products so that they are knowledgeable about Products and can represent Products in accordance with the terms of this Agreement and in compliance with Applicable Law. Company shall provide initial training to Distributor’s personnel at Company’s cost and expense as set forth in Schedule C. Distributor may, at its discretion, have Company provide additional training to its personnel at Distributor’s cost and expense as set forth in Schedule C.
5.8 Attendance at Meetings. At Company’s request, Distributor shall, at its own expense, have a representative(s) attend a sales meeting sponsored by Company at least once each year during the Term.
5.9 Technical Support. Distributor shall be responsible for training its personnel who provide technical services for the Products in the Territory or arrange to train personnel of Distributor’s Technical Support Company who will provide technical services for the Products in the Territory. In the Territory, Distributor shall itself or through its Technical Support Company maintain service personnel qualified to provide repair service and technical support for Products during its normal business hours.
5.10 Non-Compete. Distributor covenants that during [*], Distributor shall not directly or indirectly itself or through any Affiliate or licensee manufacture, promote, market, distribute or sell, or assist in the promotion, marketing, distribution or sale of any Competitive Product in the Pre-Reimbursement Approval Channel or Post-Reimbursement Approval Channel in the Territory; provided, however, that [*]
6. Company’s Duties.
6.1 Development of Products. Subject to the terms and conditions of this Agreement, Company and Distributor shall use commercially reasonable efforts to develop [*], in each case in accordance with [*]. Without limiting the generality of the foregoing, the Parties shall hold a kick-off meeting regarding development of [*] no later than [*] and work together to reach as soon as reasonably possible a mutually agreed architecture, specification, development plan and timeline for the development of [*] Development Plan”). Upon the Parties agreeing to the [*] Development Plan in writing, the Company will use its commercially reasonable efforts to develop the [*] in accordance with the [*] Development Plan and Distributor will reasonably cooperate with and provide such assistance to Company as may be set forth in the [*] Development Plan. After [*], the Parties will discuss in good faith a development agreement covering [*] under which agreement Distributor would be required to fund such development activities.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
27.
6.2 Supply of Products. Subject to the terms and conditions of this Agreement, Company shall manufacture and supply Distributor’s requirements of the Products that fully meet the Specifications and in full compliance with Applicable Law; provided that following the receipt of the 1St Reimbursement Approval and Company making NSTS available in the Territory, Company may discontinue SenStar Treatment Link.
6.3 Company Inability to Supply Products. If Company receives a written notice alleging Company’s material breach of or default under any loan or debt financing agreement (the “Default Notice”) and Distributor reasonably determines that such breach or default will result in Company not being able to supply Distributor with an amount of Product to satisfy the then-current New Sales Forecast or, if for any reason, Company is unable to supply Distributor with Products meeting at least [*], then, in addition to any other remedies available to Distributor at law or in equity or otherwise pursuant to this Agreement, (a) Distributor may order directly from Company’s contract manufacturers quantities of Products sufficient to satisfy Distributor’s forecasts that Distributor continues to provide to Company in accordance with Section 3.2 as well as all Products which Company was unable to manufacture and sell to Distributor and (b) all Products that Distributor orders from Company’s contract manufacturers and all Products that Company was unable to supply and are not supplied by Company’s contract manufacturers shall count toward satisfying Distributor’s Minimum Purchase Requirement at the prices set forth in this Agreement. As soon as Company is able to demonstrate, to Distributor’s reasonable satisfaction, that Company may resume supply in a manner that complies with this Agreement, Distributor will no longer have the right to place orders for the Products with Company’s contract manufacturers. Further, until the earlier of a Change of Control of Company or Company’s securities being publicly listed on a securities exchange, Company shall provide quarterly financial statements to Distributor and immediately notify Distributor of Company’s receipt of a notice alleging Company’s material breach of or default under any loan or debt financing agreement.
6.4 Technical and Promotional Materials. Company shall furnish Distributor with Company’s technical, clinical and safety information as reasonably required by Distributor to evaluate, market, promote, distribute and sell Products in the Territory. Without limiting the generality of the foregoing, Company shall provide Distributor with one (1) copy of all Documentation electronically within [*] after the Effective Date if available as of the Effective Date or [*] after finalization by Company if not available as of the Effective Date.
6.5 Promotional Materials. Company shall furnish Distributor with Company’s brochures and other marketing materials, which Distributor may use to prepare its own advertising and promotional materials for use in the Territory.
6.6 Marketing Support. Company shall provide commercially reasonable support to Distributor in connection with Distributor’s marketing, promotion and distribution of the Products in the Territory.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
28.
6.7 Product Changes. If Company desires to change (i) the form, fit or function or (ii) any part, process or manufacturer, which change will require the approval of any of the Regulatory Authorities or would be expected to have a material impact on quality control processes or the quality of the Products, then Company shall provide Distributor with [*] prior written notice and shall obtain all Regulatory Approvals necessary to allow Distributor to import, market, promote, distribute and sell such changed Product in the Territory as a medical device for the treatment of Major Depressive Disorder indications that are substantially similar to the Major Depressive Disorder indications for which the Product is then approved in the Territory. Company shall not discontinue any Product unless (a) Company provides Distributor with at least six (6) months prior written notice of the discontinuance, (b) Company introduces a new Product for use in the Territory that retains reasonably comparable or better functionality as the discontinued Product and (c) ensures that repair service and compatible service parts and components necessary for after-sales service of the discontinued Products remain available until the earlier of the date that is: (i) [*] after Company discontinues any Product; or (ii) the end of the Term.
6.8 Recall. Company and the DMAH have sole authority to issue a recall or require corrective action with respect to Products in the Territory (collectively, the “Recall”) including those required by Applicable Law or a regulatory authority in the Territory. In the case of any Recall, Company and DMAH shall be responsible to plan and lead the Recall with input from the Distributor, and Distributor shall be responsible for executing the plan within the Territory including communications with customers, performing all field activities in the Territory and, if necessary, the physical return of the Product from the Territory. The Parties shall fully cooperate with each other concerning any Recall. Except to the extent that the Recall is caused by Distributor’s negligence (such improper installation or service of the Products) or breach of this Agreement, Company shall provide Distributor with replacement Products free of charge and shall reimburse Distributor for its out-of-pocket expenses and technical service labor at Distributor’s then-current list price for labor. If the Recall is caused by Distributor’s negligence or Distributor’s material breach of this Agreement, Distributor shall be responsible for the cost of replacement Products and all its expenses incurred in the Recall.
6.9 Safety Information. Company shall provide to Distributor copies of any correspondence it provides to MHLW or other regulatory authority in the Territory concerning safety issues with respect to the Products (whether experienced inside or outside the Territory) promptly after sending such correspondence to MHLW or the applicable regulatory authority.
6.10 Technical Training. Company shall provide initial technical support training to Distributor’s personnel as set forth in Schedule C at Company’s expense. Distributor may require Company to provide additional technical training to Distributor personnel to be held at a location or locations to be determined by Distributor and at Distributor’s expense as set forth in Schedule C.
6.11 Technical Support. Company shall provide technical support in accordance with Schedule K.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
29.
6.12 Distributor Audit Rights. Company shall ensure that an independent third party selected by Distributor and reasonably acceptable to Company and any Regulatory Authorities, to the extent permitted by Applicable Law, may, during regular business hours, after entering into a confidentiality agreement reasonably acceptable to Company and upon reasonable advance written notice, not more than once annually, (i) examine and inspect Company’s facilities or, subject to any third party confidentiality restrictions and other obligations, the facilities of any subcontractor and the facilities of the DMAH and Company’s Agent, and (ii) subject to Applicable Law and any third party confidentiality restrictions and other obligations, inspect all data, documentation and work product relating to the activities performed by Company, its subcontractors, DMAH and Company’s Agent, including relating to the Products and their manufacture, solely for the purposes of determining Company’s compliance with the terms of this Agreement. This right of Distributor to audit and inspect the data, documentation, and work product of the Company, its subcontractors, the DMAH and Company Agent may be exercised at any time during the Term upon reasonable notice (subject to each Party’s record retention policies then in effect), or such longer period as shall be required by Applicable Law.
7. Intellectual Property.
7.1 Company’s Registration of Trademarks. Promptly after the Effective Date, the Company shall register the English language Company Trademarks not yet registered in the Territory and the katakana Company Trademarks in the Territory and, once obtained, maintain the registration of such Company Trademarks in the Territory during the Term.
7.2 Distributor’s Use of Company Trademarks.
7.2.1 Company hereby grants to Distributor an exclusive (except for those to whom Company may sell Products in the Territory as set forth in Section 2.1), royalty-free, non-transferable, license to use the Company Trademarks during the Term to import, market, promote, distribute and sell the Products in the Pre-Reimbursement Approval Channel and Post-Reimbursement Approval Channel in the Territory, with the rights to grant sublicenses to Distributor’s Affiliates, Sales Representatives, subdistributors and customers.
7.2.2 Distributor shall brand the Products with the Company Trademarks at no cost to Distributor; provided that Distributor shall also have the right to co-brand the Products with Distributor’s trademarks approved by Company (“Distributor Trademarks”), such approval not to be unreasonably withheld. The ownership of Distributor Trademarks used to co-brand the Products shall remain with Distributor, and Company shall have no rights with respect to the Distributor Trademarks, except for a limited royalty-free license to use after termination of this Agreement by either Party any Distributor Trademarks that Distributor develops and uses solely in connection with the promotion and distribution of the Products in the Territory.
7.2.3 Distributor shall use Company Trademarks in accordance with Company’s trademark usage guidelines, a draft of which Company has provided to Distributor prior to the Effective Date and which Company may update and finalize prior to Distributor promoting sales of Products in the Territory. Company may update such guidelines from time to time on at least [*] prior written notice; provided that Distributor will not be required to destroy
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
30.
marketing collateral based on the guidelines previously in effect and already produced or under a non-cancelable contract or a contract cancelable only with a penalty or other payment obligation for production as of the date of such notice. Company shall retain all right, title and interest in and to all Company Trademarks and all usage of the Company Trademarks by Distributor shall inure to the benefit of Company, except to the extent related to the Distributor Trademarks. Distributor shall take no steps to register any Company Trademarks or any other ▇▇▇▇ confusingly similar to any of the Company Trademarks, and upon the Transition Effective Date, Distributor shall not initiate any new use of the Company Trademarks. Distributor shall not use Company Trademarks in any manner that is disparaging or that otherwise portrays Company or Products in a negative light. Company shall not apply to register the Distributor Trademarks or any trademarks that combine Distributor Trademarks and Company Trademarks in any jurisdiction.
7.3 Software.
7.3.1 Company hereby grants to Distributor a limited, non-exclusive, non-transferable, royalty-free and non-sublicensable right (except to the Technical Support Company and to customers as set forth in this Section 7.3.1) and license to: (a) use the Software to demonstrate, maintain and support the Products; and (b) supply licensed copies of the Software and grant sublicenses to use the Software to purchasers of the NeuroStar Products in the Territory solely for use with the Products or a TrakStar Computer (the “Limited License”). For the avoidance of doubt, the Limited License will remain in effect after expiration or termination of this Agreement solely to allow Distributor’s customers to continue using Products purchased prior to expiration or termination of the Agreement. Distributor obtains no right, title or interest in or to the Software, except for the Limited License granted pursuant hereto, and Company and its licensors reserve all rights not expressly granted.
7.3.2 Distributor or the Technical Support Company shall be responsible for loading the TrakStar Software onto the TrakStar Computer and for loading all new versions, releases updates and bug fixes to the Software released after the applicable Product is placed into inventory at Company’s warehouse. Company shall supply to Distributor or the Technical Support Company, as directed by Distributor, all new versions, releases, updates and bug fixes to the Software in such format as to enable the Distributor or Technical Support Company to install such new version, release, update or bug fix in the Products purchased by Distributor’s customers in the Territory and the TrackStar Computer, as applicable.
7.3.3 Except as set forth in this Section 7.3, Distributor may not loan, rent, lease, license or otherwise transfer to any other person, or host on behalf of any other person, the Software, and may not copy, modify, remove, disintegrate, create derivative works from or tamper with the Software.
7.3.4 Distributor shall require all purchasers of the NeuroStar Product in the Territory to enter in an agreement of sale that contains at least the terms attached to Schedule I for which Distributor may prepare a Japanese version that is revised so as to be enforceable in accordance with the laws of the Territory and in accordance with local custom and practice (the “Minimum Terms and Conditions of Sale”). Company may update the Minimum Terms and
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
31.
Conditions of Sale from time to time on at least [*] prior written notice to Distributor. Company shall consider in good faith any comments that Distributor may have regarding any updates to the Minimum Terms and Conditions of Sale. Distributor shall update its terms and conditions of sale for the NeuroStar Product as promptly as possible after expiration of such [*] so as to be consistent with the updated Minimum Terms and Conditions of Sale, enforceable in accordance with the laws of the Territory and in accordance with local custom and practice and ensure that all new customers for the Products after the completion of the notice period (that is, customers who have not previously purchased Products from Distributor and who are not purchasing through a purchasing cooperative or other entity that already has a contract with Distributor for the purchase of Products) enter into an agreement of sale that includes at least the updated Minimum Terms and Conditions of Sale as modified to be in accordance with the laws of the Territory and in accordance with local custom and practice.
7.3.5 Except as expressly permitted under Applicable Law, Distributor may not decompile, reverse engineer or disassemble the Software and may not disintegrate the Redistributable Code from the Software.
7.4 Documentation. Distributor may copy and distribute the Documentation as necessary to maintain, support and service the Products; provided that it shall maintain all Company Trademarks and copyright notices thereon. Company hereby grants to Distributor a limited, royalty-free, non-exclusive, non-transferable, and non-sublicensable right and license (except to the Technical Support Company) to: (a) copy, use and distribute the Documentation to demonstrate, maintain, support and service the Products; and (b) create Japanese versions of the Documentation and copy, use and distribute such translated versions of the Documentation to demonstrate, maintain, support and service the Products and supply such Documentation to the Technical Support Company and purchasers of the Product in the Territory. Distributor obtains no right, title or interest in or to the Documentation, except as expressly set forth in this Section 7.4, and Company reserves all rights not expressly granted.
7.5 Marketing Materials. Company may from time to time provide Distributor with certain materials such as, but not limited to: demonstration Products, models, advertising materials, booklets and brochures, reprints of technical articles and marketing plans (the “Marketing Materials”). The Marketing Materials shall remain the sole property of Company and any such Marketing Materials remaining in the possession of Distributor shall be promptly destroyed, at Distributor’s expense, upon request any time after the expiration of this Agreement; provided that Distributor may continue to use the Marketing Materials and will not be required to destroy the Marketing Materials until Distributor’s rights under Section 13.2 expire. Distributor shall also have the right to cross link to Company’s website and to use Company Trademarks on its website to promote and sell the Products in the Territory.
7.6 Intellectual Property Rights. Except as set forth in this Section 7, Distributor shall have no rights to any intellectual property rights of Company.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
32.
8. Confidential Information.
8.1 Confidential Information. As used in this Agreement, the term “Confidential Information” means all information, whether it be written or oral, including all production schedules, lines of products, volumes of business, processes, new product developments, product designs, formulae, technical information, laboratory data, clinical data, patent information, know-how, trade secrets, financial and strategic information, marketing and promotional information and data, and other material relating to any products, projects or processes of one Party (the “Disclosing Party”) that is provided to, or otherwise obtained by, the other Party (the “Receiving Party”) in connection with this Agreement. Notwithstanding the foregoing sentence, Confidential Information shall not include any information or materials that:
8.1.1 were already known to the Receiving Party (other than under an obligation of confidentiality), at the time of disclosure by the Disclosing Party, to the extent such Receiving Party has documentary evidence to that effect;
8.1.2 were generally available to the public or otherwise part of the public domain at the time of disclosure thereof to the Receiving Party;
8.1.3 became generally available to the public or otherwise part of the public domain after disclosure or development thereof, as the case may be, and other than through any act or omission of a Party in breach of such Party’s confidentiality obligations under this Agreement;
8.1.4 were disclosed to a Party, other than under an obligation of confidentiality, by a third party who had no obligation to the Disclosing Party not to disclose such information to others; or
8.1.5 were independently discovered or developed by or on behalf of the Receiving Party without the use of the Confidential Information belonging to the other Party, to the extent such Receiving Party has documentary evidence to that effect.
8.2 Confidentiality Obligations. Each of Distributor and Company shall keep all Confidential Information received from or on behalf of the other Party with the same degree of care with which it maintains the confidentiality of its own Confidential Information, but in all cases no less than a reasonable degree of care. Neither Party shall use such Confidential Information for any purpose other than in performance of its obligations or the exercise of its rights pursuant to this Agreement or disclose the same to any other person other than to such of its and its Affiliates’ directors, managers, employees, independent contractors, agents, consultants or sublicensees who have a need to know such Confidential Information to implement the terms of this Agreement or enforce its rights under this Agreement; provided, however, that a Receiving Party shall advise any of its and its Affiliates’ directors, managers, employees, independent contractors, agents, consultants or sublicensees who receives such Confidential Information of the confidential nature thereof and of the obligations contained in this Agreement relating thereto, and the Receiving Party shall ensure (including, in the case of a third party, by means of a written agreement with such third party having terms at least as protective as those contained in this Section 8) that all such directors, managers, employees, independent contractors, agents, consultants or sublicensees comply with such obligations. Upon
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
33.
expiration or termination of this Agreement, the Receiving Party shall return or destroy all documents, tapes or other media containing Confidential Information of the Disclosing Party that remain in the possession of the Receiving Party or its directors, managers, employees, independent contractors, agents, consultants or sublicensees, except that the Receiving Party may keep one copy of the Confidential Information in the legal department files of the Receiving Party, solely for archival purposes. Such archival copy shall be deemed to be the property of the Disclosing Party, and shall continue to be subject to the provisions of this Section 8. It is understood that receipt of Confidential Information under this Agreement will not limit the Receiving Party from assigning its employees to any particular job or task in any way it may choose, subject to causing such employees to comply with this Section 8.
8.3 Permitted Disclosure and Use. Notwithstanding Section 8.2 either Party may disclose Confidential Information belonging to the other Party to the extent such disclosure is reasonably necessary to: (a) comply with or enforce any of the provisions of this Agreement, (b) comply with Applicable Law or (c) to the extent such disclosure is reasonably necessary to obtain or maintain regulatory approval of a Product, to the extent such disclosure is made to a Governmental Authority. If a Receiving Party deems it necessary to disclose Confidential Information of the Disclosing Party pursuant to this Section 8.3, the Receiving Party shall give reasonable advance written notice of such disclosure to the Disclosing Party to permit the Disclosing Party sufficient opportunity to object to such disclosure or to take measures to ensure confidential treatment of such information, including seeking a protective order or other appropriate remedy. Notwithstanding Section 8.2, Distributor may also disclose Confidential Information belonging to Company related to Product (i) to third parties in connection with the promotion, marketing and sales of Products in the Territory and (ii) to potential subdistributors and potential Sales Representatives (provided that such third parties in clauses (i) and (ii) are bound by written agreements having terms at least as protective as those contained in this Section 8 with respect to keeping such Confidential Information confidential). The Receiving Party shall notify the Disclosing Party promptly upon discovery of any unauthorized use or disclosure of the Disclosing Party’s Confidential Information, and will cooperate with the Disclosing Party in any reasonably requested fashion to assist the Disclosing Party to regain possession of such Confidential Information and to prevent its further unauthorized use or disclosure.
8.4 Notification. In the event that a Receiving Party becomes aware that a third party recipient of Confidential Information has breached its confidentiality obligations, then the Receiving Party shall promptly inform the Disclosing Party of such event, and the Parties will cooperate in their investigation of such occurrence and enforcement of the provisions of the relevant confidentiality agreement.
8.5 Publicity; Filing of this Agreement. Each Party shall maintain the confidentiality of all provisions of this Agreement, and without the prior written consent of the other Party, which consent shall not be unreasonably withheld, neither Party nor its respective Affiliates shall make any press release or other public announcement of or otherwise publicly disclose the provisions of this Agreement to any third party, except for: (a) disclosures required any national securities exchange and any other disclosures made pursuant to any listing agreement with a national securities exchange, in which case the disclosing Party shall provide the nondisclosing
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
34.
Party with at least [*] notice unless otherwise not practicable, but in any event no later than the time the disclosure required by the regulations of the applicable national securities exchange or listing agreement is made, and (b) disclosures as may be required by Applicable Law, in which case the disclosing Party shall provide the nondisclosing Party with prompt advance notice of such disclosure and cooperate with the nondisclosing Party to seek a protective order or other appropriate remedy, including a request for confidential treatment in the case of Company for a filing with the Securities and Exchange Commission; and (c) other disclosures for which consent has previously been given. A Party may publicly disclose without regard to the preceding requirements of this Section 8.5 any information that was previously publicly disclosed pursuant to this Section 8.5.
8.6 Use of Names. Except as otherwise set forth in this Agreement, neither Party shall use the name of the other Party in relation to this transaction in any public announcement, press release or other public document without the written consent of such other Party, which consent shall not be unreasonably withheld.
8.7 No Implied Licenses. All right, title and interest in and to the Disclosing Party’s Confidential Information, including, without limitation, any intellectual property rights related thereto, shall remain with the Disclosing Party, subject to the limited licenses or rights to use granted to the Receiving Party in this Agreement. Except as otherwise provided in this Agreement, neither this Agreement, nor the cooperation of the Parties during the Term, shall be deemed to grant to the Receiving Party any right or licenses, express or implied, under any patents or patent applications, or to use or practice any know-how, technology or inventions, owned or controlled by Disclosing Party. Nothing in this Agreement shall be construed to prevent the Disclosing Party from exploiting the Disclosing Party’s Confidential Information and all patent and other intellectual property rights therein and appurtenant thereto in any manner for any purpose.
8.8 Survival. The obligations and prohibitions contained in this Section 8 as they apply to Confidential Information shall survive the expiration or termination of this Agreement for a period of [*].
9. Representations and Warranties.
9.1 Mutual Representations and Warranties. Each Party hereby represents and warrants to the other Party as follows, as of the Effective Date:
9.1.1 Corporate Existence and Power. It is a company or corporation duly organized, validly existing, and in good standing (only in the case of Company) under the laws of the jurisdiction in which it is incorporated and has full corporate power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted and as contemplated in this Agreement, including the right to grant the licenses granted by it hereunder.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
35.
9.1.2 Authority and Binding Agreement. (a) It has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder, (b) it has taken all necessary corporate action on its part required to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder, and (c) this Agreement has been duly executed and delivered on behalf of such Party and constitutes a legal, valid, and binding obligation of such Party that is enforceable against it in accordance with its terms, except as enforcement may be affected by bankruptcy, insolvency or other similar laws and by general principles of equity.
9.1.3 No Conflicts. The execution, delivery and performance of this Agreement in accordance with its terms by it does not (a) conflict with any agreement, instrument or understanding, oral or written, to which it is a party and by which it may be bound or (b) violate any Applicable Law.
9.1.4 All Consents and Approvals Obtained. Except with respect to the Regulatory Approvals and Distributor Approvals, (a) all necessary consents, approvals and authorizations of, and (b) all notices to, and filings with all Governmental Authorities and third parties required to be obtained or provided by such Party in connection with the execution, delivery and performance of this Agreement have been obtained.
9.2 Additional Representations, Warranties and Covenants of Company. Company hereby represents, warrants and covenants to Distributor that:
9.2.1 To the knowledge of Company, the design, manufacture, marketing, sale, offer for sale, importation and use of the Products, Software and the System as of the Effective Date for treatment of Major Depressive Disorder do not infringe or misappropriate the intellectual property rights of any third party.
9.2.2 No claim has been made to Company in writing which alleges that the design, manufacture, marketing, sale, offer for sale, importation or use of the Products, Software or the System for treatment of Major Depressive Disorder infringes or misappropriates the intellectual property rights of a third party.
9.2.3 Except with respect to the Redistributable Code, Company is the sole legal and beneficial owner of all Software and intellectual property rights related thereto, free and clear of any liens or other encumbrances.
9.2.4 Company’s agreements with all third parties for the Redistributable Code are in full force and effect, are enforceable in accordance with their terms by Company and, to Company’s knowledge, the third parties thereto and such agreements will not cease to be so valid and binding and in full force and effect as a result of the transactions contemplated by this Agreement.
9.2.5 Company is the sole legal and beneficial owner of the Company Trademarks, free and clear of any liens or other encumbrances. To Company’s knowledge (a) there is no reason that would prevent Company from registering the katakana Company Trademarks in the Territory in the IC classes indicated in Schedule D and (b) no third party is using (other than [*]) or infringing the Company Trademarks in the Territory. Company has not entered (other than a non-exclusive license agreement with [*]) nor during the Term will enter
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
36.
into any agreement granting any right, interest or claim in or to, any Company Trademarks for use in connection with the promotion, import into the Territory, distribution or sale of Products in the Pre-Reimbursement Approval Channel or Post-Reimbursement Approval Channel to any third party other than non-exclusive license agreements with Eye-Lens and/or [*] that would not interfere with or impair rights granted to Distributor pursuant to this Agreement.
9.2.6 There is no pending or, to Company’s knowledge, threatened legal, administrative, arbitral or other proceeding, claim, suit or action against or, to the knowledge of the Company, any governmental or regulatory investigation of Company, nor any injunction, order, judgment, ruling or decree imposed upon or, to Company’s knowledge, threatened to be imposed upon Company involving the Products, System, Software, Trademarks or Company’s failure to comply with Applicable Law with respect to the Products or that would potentially prevent Company from fulfilling its obligations set forth in this Agreement.
9.2.7 Neither Company, its Affiliates nor, to Company’s knowledge, its shareholders have entered into any agreement for, or are currently in any negotiations for, a Change of Control of Company.
9.2.8 Company’s agreements with all contract manufacturers for the Products are in full force and effect, are enforceable in accordance with their terms by Company and, to Company’s knowledge, the third parties thereto and such agreements will not cease to be so valid and binding and in full force and effect as a result of the transactions contemplated by this Agreement.
9.2.9 Company has obtained the written agreement of Company’s contract manufacturers to accept and honor all orders placed by Distributor pursuant to Section 6.3.
9.2.10 To Company’s knowledge, all work related to obtaining the Regulatory Approvals has been performed in accordance with all Applicable Law.
9.2.11 Company has a valid Foreign Manufacturer Accreditation in respect of the Products and will maintain the Foreign Manufacturer Accreditation during the Term of this Agreement.
9.3 Limited Product Warranty.
9.3.1 Company warrants to Distributor that each unit of Product sold to Distributor under this Agreement and the Software distributed in connection therewith shall: (a) at the time of delivery be free of liens, claims and encumbrances; (b) at the time of delivery comply with the Specifications; and (c) be free from Defects until the earlier of [*] after the date of installation of such unit of the Product at the end-user customer and [*] after delivery of such unit of the Product to Distributor pursuant to Section 3.7.1 (for the applicable unit of the Product, the “Warranty Period”). The foregoing warranties set forth in clause (c) of this Section 9.3.1 apply only if Products have not been mishandled, damaged or altered after delivery of the Products to Distributor pursuant to Section 3.7.1. All claims in respect of the warranty set forth in the foregoing sentence must be made no later than the Warranty Period, and Company shall have no responsibility for claims for breach of the warranty set forth in this Section 9.3.1 made
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
37.
after expiration of the applicable Warranty Period. Notwithstanding anything to the contrary in this Agreement: (i) the warranties set forth in this Section 9.3.1 shall not apply to any Defect caused by improper installation or Distributor’s or its customer’s failure to maintain such Products in accordance with documentation provided by Company to Distributor sufficiently prior to such failure so as to have allowed Distributor or Distributor’s customer a reasonably opportunity to have avoided such failure, provided that such documentation complies with Applicable Law and the provisions of this Agreement; and (ii) Company shall not be obligated to furnish service under such warranty: (1) to repair damage resulting from attempts by personnel other than Company representatives to repair Products, except for personnel of Distributor or the Technical Service Company who have attempted to repair damage in accordance with documentation provided by Company to Distributor or the Technical Service Company and caused no additional damage or (2) to repair damage resulting from connection to incompatible equipment or use other than as set forth in documentation provided by Company to Distributor prior to the occurrence of the Defect.
9.3.2 If during the Warranty Period for a particular unit of Product (other than the Software), Distributor claims that a Product or component thereof does not comply with the warranties set forth in Section 9.3.1, Distributor shall return the allegedly defective Product(s) or component to Company in accordance with Section 3.10; provided that (a) units of NeuroStar Product that experience an Out of Box Failure may not be returned to Company except in accordance with Section 9.3.5 and (b) for the avoidance of doubt, in the case of alleged defects that can be addressed by replacement of a part or a field replacement unit, only the alleged defective part or field replacement unit may be returned to Company. Upon confirmation by Company that the Product(s) do not comply with the foregoing warranties and that the Warranty Period for Product(s) did not expire prior to Distributor making the warranty claim, Company shall at its option and expense and, as Distributor’s sole and exclusive remedy for Company’s breach of the warranty set forth in Section 9.3.1, repair or replace the defective Product(s) and ship the repaired or replaced Product(s) to Distributor at Company’s expense, or credit Distributor’s account for such Product(s), provided, however that notwithstanding any other provision of this Agreement, Company itself shall have no obligation to provide repair or other labor services with personnel located in the Territory (including any labor to uninstall or install any parts). As to alleged Defective Software, upon confirmation by the Company that the Software does not comply with the warranties set forth in Section 9.3.1, Company shall as Distributor’s sole and exclusive remedy for Company’s breach of the warranties set forth in Section 9.3.1 promptly provide Distributor with a work around, bug fix, patch or other support services that restores operation of the Software to be in substantial compliance with the Software Documentation. If Company determines that the Product(s) or Software comply with the warranties set forth in Section 9.3.1 or that the Warranty Period for the Product(s) expired prior to Distributor making the warranty claim, Company shall notify Distributor of such fact and provide to Distributor a report setting forth the analysis and reasoning supporting such determination by the Company (“Warranty Report”). In such a case Company shall have no obligation to provide warranty service and shall instead comply with Distributor’s direction regarding the disposition of such Product at Distributor’s expense. For the avoidance of doubt, the provisions of this Section 9.3.2 shall in no way limit Company’s indemnification obligations set forth in Section 10.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
38.
9.3.3 If Distributor disagrees with the conclusions set forth in Company’s Warranty Report, then each Party shall appoint a senior manager to meet and discuss in good faith whether the returned Products contain any Defects. If such senior managers are unable to reach agreement on whether the returned Products contain any Defects within a period of [*], then either Party may request that an independent third party expert reasonably acceptable to the other Party review the Warranty Report and the returned Products, determine whether the Products contain any Defects and issue a written report to both Parties describing the review conducted by the third party expert, the determination of whether the returned Products contain any Defects and the reasons for the third party experts determination. The costs of the third party expert shall be equally borne by each Party. If Company agrees with Distributor as to the existence of Defects in the returned Products, the results of Company’s analysis of the returned Products confirms the existence of a Defect in the Products or the third party expert concludes that there is a Defect in the returned Products and in all cases that the Warranty Period for the Product(s) did not expire prior to Distributor making the warranty claim, then Company will comply with the remedies set forth in Section 9.3.1.
9.3.4 The cost of out-of-warranty repairs is subject to Company’s then-current charges. Distributor must authorize such charges prior to Company performing any such repairs. All charges shall be authorized by Distributor issuing a purchase order therefor.
9.3.5 In the case of an Out of Box Failure, Distributor or its Technical Support Company will, in accordance with the procedures in Schedule K, contact Company’s technical support department and follow all steps recommended by Company to address the Out of Box Failure such that all non-conformances and failures are corrected and the NeuroStar OUS Installation Record ([*]) can be completed showing all items and criteria thereon as complete and satisfied. [*] If after following all steps recommended by Company to address the Out of Box Failure, a non-conformance or failure continues to prevent Distributor or its Technical Support Company from completing the NeuroStar OUS Installation Record ([*]) showing all items and criteria thereon as complete and satisfied, then Distributor shall return the allegedly defective NeuroStar Starter Package to Company in accordance with Section 3.10.
9.3.6 Following Distributor’s [*] of NeuroStar Starter Packages at customer locations, Distributor and Company will discuss in good faith the visual inspection procedure set forth on Schedule E and whether and what adjustments to such procedures should be made to assure high customer satisfaction at time of installation.
10. Indemnification.
10.1 Indemnification.
10.1.1 Notwithstanding anything to the contrary contained herein, Distributor shall indemnify, defend and hold Company and its directors, officers, employees and agents harmless from and against any and all losses, damages, liabilities, costs and expenses (including reasonable attorneys’ fees and expenses) (collectively, “Losses”) arising in connection with any and all charges, complaints, actions, suits, proceedings, hearings, investigations, claims, demands, judgments, orders, decrees, stipulations or injunctions by a third party (each a “Third
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
39.
Party Claim”) that: (a) seeks recovery of Losses claimed by the employees or agents of Distributor or its Affiliates; (b) asserts breach of warranties made by Distributor or its Affiliates to purchasers of the Products in the Territory different from or in addition to those made in by Company in this Agreement with respect to the Products; and/or (c) seeks recovery of damages for injury to persons or damage to property or any other liabilities caused by the willful act, recklessness or negligence of Distributor or its Affiliates, in each case except to the extent that such Losses are subject to indemnification by the Company pursuant to Section 10.1.2.
10.1.2 Notwithstanding anything to the contrary contained herein, Company shall indemnify, defend and hold Distributor and its directors, officers, employees and agents harmless from and against any and all Losses and arising in connection with any and all Third Party Claims resulting or otherwise arising from: (a) any personal injuries, illness, death and/or property damages resulting from any Defective Product, which for purposes of this Section
10.1.3 shall include design defects in the Products; (b) any infringement of third party intellectual property rights by the import, marketing, promotion, sale or use of the Products, System, Software or Company Trademarks in the Territory in accordance with this Agreement; and (c) the negligence, recklessness or willful misconduct of Company and/or the DMAH, in each case except to the extent that such Losses are subject to indemnification by Distributor pursuant to Section 10.1.1.
10.1.4 In no event shall Company have any obligation or liability to Distributor under this Section 10.1 for any Losses suffered by a third party as a result of:
(a) Distributor or its Affiliates making any warranty, express or implied, to customers that is different from Company’s warranty set forth in Section 9.3.1;
(b) Any design, cosmetic or functional change in Product made intentionally by Distributor or its Affiliates, except as Distributor may do so in accordance with the Documentation or pursuant to operation of the Software;
(c) Distributor or its Affiliates not storing, handling, or transporting the Products in accordance with the Documentation provided by Company to Distributor or service performed by or on behalf of Distributor or any of its Affiliates not in accordance with Documentation provided to Distributor by Company; or
(d) Distributor or its Affiliates labeling or relabeling Products as any other product or a component of any other product, except that Distributor or its Affiliates may attach labels to Products in compliance with Applicable Law in the Territory.
10.2 Indemnification Procedures.
10.2.1 Notice of Claim. All indemnification claims in respect of any indemnitee seeking indemnity under Section 10.1.1 or 10.1.2, as applicable (collectively, the “Indemnitees” and each an “Indemnitee”) will be made solely by the corresponding Party (the “Indemnified Party”). The Indemnified Party will give the indemnifying Party (the “Indemnifying Party”) prompt written notice (an “Indemnification Claim Notice”) of any Losses and any legal
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
40.
proceeding initiated by a Third Party against the Indemnified Party as to which the Indemnified Party intends to make a request for indemnification under 10.1.1 or 10.1.2, as applicable, but in no event will the Indemnifying Party be liable for any Losses that result from any delay in providing such notice which materially prejudices the defense of such proceeding. Each Indemnification Claim Notice shall contain a description of the claim and the nature and amount of such Loss (to the extent that the nature and amount of such Loss are known at such time). Together with the Indemnification Claim Notice, the Indemnified Party will furnish promptly to the Indemnifying Party copies of all notices and documents (including court papers) received by any Indemnitee in connection with the Third Party Claim.
10.2.2 Control of Defense. At its option, the Indemnifying Party may assume the defense of any Third Party Claim subject to indemnification as provided for in 10.1.1 or 10.1.2, as applicable, by giving written notice to the Indemnified Party within thirty (30) days after the Indemnifying Party’s receipt of an Indemnification Claim Notice. Upon assuming the defense of a Third Party Claim, the Indemnifying Party may appoint as lead counsel in the defense of the Third Party Claim any legal counsel it selects, and such Indemnifying Party shall thereafter continue to defend such Third Party Claim in good faith. Should the Indemnifying Party assume the defense of a Third Party Claim (and continue to defend such Third Party Claim in good faith), the Indemnifying Party will not be liable to the Indemnified Party or any other Indemnitee for any legal expenses subsequently incurred by such Indemnified Party or other Indemnitee in connection with the analysis, defense or settlement of the Third Party Claim, unless the Indemnifying Party has failed to assume the defense and employ counsel in accordance with this Section 10.2.2.
10.2.3 Right to Participate in Defense. Without limiting Section 10.2.2, any Indemnitee will be entitled to participate in the defense of a Third Party Claim for which it has sought indemnification hereunder and to employ counsel of its choice for such purpose; provided, however, that such employment will be at the Indemnitee’s own expense unless (a) the employment thereof has been specifically authorized by the Indemnifying Party in writing, or (b) the Indemnifying Party has failed to assume the defense (or continue to defend such Third Party Claim in good faith) and employ counsel in accordance with Section 10.2.2, in which case the Indemnified Party will be allowed to control the defense.
10.2.4 Settlement. With respect to any Losses relating solely to the payment of money damages in connection with a Third Party Claim and that will not result in the Indemnitee becoming subject to injunctive relief and as to which the Indemnifying Party will have acknowledged in writing the obligation to indemnify the Indemnitee hereunder, the Indemnifying Party will have the sole right to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Loss, on such terms as the Indemnifying Party, in its reasonable discretion, will deem appropriate (provided, however, that such terms shall include a complete and unconditional release of the Indemnified Party from all liability with respect thereto), and will transfer to the Indemnified Party all amounts which said Indemnified Party will be liable to pay prior to the time of the entry of judgment. With respect to all other Losses in connection with Third Party Claims, where the Indemnifying Party has assumed the defense of the Third Party Claim in accordance with Section 10.2.2, the Indemnifying Party will have authority to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Loss,
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
41.
provided it obtains the prior written consent of the Indemnified Party (which consent will be at the Indemnified Party’s reasonable discretion). The Indemnifying Party that has assumed the defense of (and continues to defend) the Third Party Claim in accordance with Section 10.2.2 will not be liable for any settlement or other disposition of a Loss by an Indemnitee that is reached without the written consent of such Indemnifying Party. Regardless of whether the Indemnifying Party chooses to defend or prosecute any Third Party Claim, no Indemnitee will admit any liability with respect to, or settle, compromise or discharge, any Third Party Claim without first offering to the Indemnifying Party the opportunity to assume the defense of the Third Party Claim in accordance with Section 10.2.2.
10.2.5 Cooperation. If the Indemnifying Party chooses to defend or prosecute any Third Party Claim, the Indemnified Party will, and will cause each other Indemnitee to, cooperate in the defense or prosecution thereof and will furnish such records, information and testimony, provide such witnesses and attend such conferences, discovery proceedings, hearings, trials and appeals as may be reasonably requested in connection with such Third Party Claim. Such cooperation will include access during normal business hours afforded to the Indemnifying Party to, and reasonable retention by the Indemnified Party of, records and information that are reasonably relevant to such Third Party Claim, and making Indemnitees and other employees and agents available on a mutually convenient basis to provide additional information and explanation of any material provided hereunder, and the Indemnifying Party will reimburse the Indemnified Party for all its reasonable out-of-pocket expenses incurred in connection with such cooperation.
10.2.6 Expenses of the Indemnified Party. Except as provided above, the reasonable and verifiable costs and expenses, including fees and disbursements of counsel, incurred by the Indemnified Party in connection with any Third Party Claim will be reimbursed on a calendar quarter basis by the Indemnifying Party, without prejudice to the Indemnifying Party’s right to contest the Indemnified Party’s right to indemnification and subject to refund in the event the Indemnifying Party is ultimately held not to be obligated to indemnify the Indemnified Party.
11. Risk Allocation.
11.1 Disclaimer. EXCEPT FOR THE REPRESENTATIONS AND WARRANTIES SET FORTH IN SECTIONS 9.1, 9.2 AND 9.3, COMPANY MAKES NO OTHER WARRANTIES OR REPRESENTATIONS, EXPRESS OR IMPLIED, STATUTORY OR OTHERWISE, AND COMPANY AND ITS THIRD PARTY SUPPLIERS (IF ANY) HEREBY DISCLAIM ALL OTHER WARRANTIES, INCLUDING, WITHOUT LIMITATION, THE IMPLIED WARRANTIES OF FITNESS FOR A PARTICULAR PURPOSE, MERCHANTABILITY, AND NON-INFRINGEMENT.
11.2 Limitation of Liability.
11.2.1 WITH THE EXCEPTION OF LIABILITY FOR INDEMNIFICATION UNDER SECTION 10 OR LIABILITY RESULTING FROM INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS, GROSS NEGLIGENCE, WILLFUL MISCONDUCT
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
42.
OR BREACH OF SECTION 8, NEITHER PARTY (THE FIRST PARTY) SHALL BE LIABLE TO THE OTHER PARTY OR ANY THIRD PARTY FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, INDIRECT, PUNITIVE OR OTHER SIMILAR DAMAGES (INCLUDING, WITHOUT LIMITATION, ANY LOST PROFITS OR LOST REVENUES) UNDER ANY THEORY OF LIABILITY. THIS LIMITATION SHALL APPLY EVEN WHERE THE FIRST PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
11.2.2 WITH THE EXCEPTION OF LIABILITY FOR INDEMNIFICATION UNDER SECTION 10 OR LIABILITY RESULTING FROM INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS, GROSS NEGLIGENCE, WILLFUL MISCONDUCT OR BREACH OF SECTION 8, IN NO EVENT SHALL COMPANY’S LIABILITY TO DISTRIBUTOR, REGARDLESS OF THE THEORY OF LIABILITY, EXCEED THE GREATER OF (A) US$5 MILLION OR (B) THE AMOUNTS RECEIVED BY COMPANY FROM DISTRIBUTOR UNDER THIS AGREEMENT (EXCLUSIVE OF REIMBURSEMENT OF PASS THROUGH EXPENSES SUCH AS SHIPPING COSTS AND DUTIES) IN THE TWELVE (12) MONTHS PRIOR TO THE ACCRUAL OF THE CLAIM.
11.3 Insurance. Each Party shall procure and maintain insurance, including comprehensive general public liability insurance and product liability insurance, and, in the case of Company clinical trial insurance, adequate and appropriate to cover its obligations hereunder. Certificates of insurance evidencing such coverage will be made available to the other Party upon written request.
12. Term and Termination.
12.1 Term. The term of this Agreement shall commence on the Effective Date and extend up to and until the end of the seventh (7th) Fiscal Year after Company receives the Product Approval, subject to earlier termination as provided below in this Section 12 (the “Initial Term”). The term of this Agreement shall be automatically extended for additional periods of two (2) Fiscal Years each such that the remaining term of the Agreement is four (4) years (the Initial Term, as so extended from time to time, the “Term”) unless either Party provides the other Party with written notice of non-extension not later than two (2) years prior to the end of the Term; provided, however, that if during the Initial Term Distributor purchases Products from Company totaling at least one hundred percent (100%) of the Dollar value set forth in the Initial Sales Forecast plus one hundred percent (100%) of the Dollar value set forth in each of the New Sales Forecasts for each subsequent Fiscal Year of the Initial Term, or during the first two (2) Fiscal Years of each extension of the Term, Distributor purchases Products from the Company totaling one hundred percent (100%) of the Dollar value set forth in each New Sales Forecast for such two (2) Fiscal Years, then (a) any previous notice of non-extension provided by Company shall be null and void and (b) the Term will be automatically extended for an additional two (2) Fiscal Years.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
43.
12.2 Termination for Cost-Sharing, Safety or Use Survey.
12.2.1 Notwithstanding any other provision of this Agreement, Distributor may terminate this Agreement at any time if Distributor reasonably believes that it is not commercially reasonable for Distributor to continue to distribute the Products in the Territory for reasons including, but not limited to, safety, efficacy, an unfavorable Reimbursement Rate (which must be below [*] to be considered unfavorable), introduction of competitive product(s), by providing prior written notice to Company and a reasonable explanation and documentation to Company that supports Distributor’s belief. Upon receipt of such written notice and explanation with documentation, Company and Distributor shall discuss the matter in good faith for a period of ninety (90) days. If at the end of such ninety (90) day period Distributor and Company cannot agree on whether it is commercially reasonable for Distributor to continue to distribute the Products in the Territory, or cannot agree on modifications to this Agreement that would make it commercially reasonable for Distributor to continue to distribute the Products, including revisions to the Minimum Purchase Requirement, True-Up Payment and/or transfer prices, then Distributor may terminate this Agreement by providing one (1) year prior written notice to Company, and no Minimum Purchase Requirement or True-Up Payment shall apply during such one (1) year period but the Minimum Purchase Requirement for the period prior to notice of termination shall be satisfied or True-Up Payment made and Initial Milestone Payment 2 and Milestone Payment 2 shall not be adjusted or refunded.
12.2.2 Notwithstanding any other provision of this Agreement, Distributor may immediately terminate this Agreement if notwithstanding the exercise of its commercially reasonable efforts Company does not obtain a use results survey period (exclusivity) of at least forty eight (48) months in connection with the first Regulatory Approvals; provided that Distributor sends notice of termination to Company within thirty (30) days after Distributor receives written notice from Company that the use results survey period (exclusivity) is less than forty eight (48) months. If Distributor terminates this Agreement under this Section 12.2.2, then the Agreement will remain in effect for one year after Distributor sends notice to Company, and no Minimum Purchase Requirement or True-Up Payment shall apply during such one year period, provided, however, that notwithstanding Section 2.1.1 Distributor will become a non-exclusive distributor during such one year period, and the restrictions on Company promoting or selling Products in the Territory in the Pre-Reimbursement Approval Channel and the Post-Reimbursement Approval Channel set forth in Sections 2.1.1, 2.1.3 and 2.1.5 shall not apply during such one year period.
12.3 Termination for Breach. Subject to the other rights of termination set forth in this Agreement, either Party may terminate this Agreement at any time in the event the other Party breaches this Agreement upon prior written notice to the breaching Party describing the breach in detail and giving the breaching Party thirty (30) days to cure the breach. If the breach has not been cured within thirty (30) days of receipt of such notice, the Party giving such notice may immediately terminate this Agreement upon notice to the breaching Party. If a breaching Party who has been given written notice and cured a breach subsequently commits the same breach within six (6) months of such earlier breach, then the other non-breaching Party shall have the right to immediately terminate this Agreement by providing notice to the breaching Party and the breaching Party shall have no further opportunity to cure.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
44.
12.4 Termination for Failure to Achieve the Product Approval. Either Party may terminate this Agreement immediately by providing prior notice to the other Party in the event that, notwithstanding the exercise of its commercially reasonable efforts, Company does not obtain the first Product Approval and the DMAH within one (1) year after the Effective Date.
12.5 Termination for Failure to Achieve the Distributor Approvals. Company may terminate this Agreement at time by providing sixty (60) days prior written notice to Distributor in the event Distributor does not obtain the Distributor Approvals within ninety (90) days after the Effective Date or at any time fails to maintain the Distributor Approvals. If Company terminates this Agreement under this Section 12.5 due to Distributor’s failure to maintain the Distributor Approvals, then Initial Milestone Payment 2 and Milestone Payment 2 shall not be adjusted or refunded and no Minimum Purchase Requirement or True-Up Payment obligations shall apply to such sixty (60) day period (but shall apply with respect to prior periods on a pro rata basis).
12.6 Termination for Failure to Achieve the 1st Reimbursement Approval.
12.6.1 Distributor may terminate this Agreement by providing written notice to Company if, notwithstanding the exercise of its commercially reasonable efforts, Company does not obtain the 1st Reimbursement Approval by the Reimbursement Approval Deadline. Distributor must provide such written notice to Company no later than ninety (90) days after the Reimbursement Approval Deadline for such termination to be effective and if Distributor does not provide such written notice with such ninety (90) day period, then Company may terminate this Agreement on written notice to Distributor. Upon a Party giving notice of termination under this Section 12.6.1, this Agreement shall terminate one (1) year from the date of the notice of termination and, for the avoidance of doubt, Initial Milestone Payment 2 and Milestone Payment 2 shall not be made and no Minimum Purchase Requirement shall apply during such one (1) year period.
12.6.2 If Distributor does not obtain the 1st Reimbursement Approval by the end of the Distributor 1st MHLW Lead Period or if during the Distributor 1st MHLW Lead Period Distributor decides to cease its commercially reasonable efforts to obtain the 1st Reimbursement Approval, then either Party may terminate this Agreement by providing written notice to the other Party. If a Party terminates this Agreement under this Section 12.6.2, then this Agreement shall terminate one (1) year from the date of the Party’s written notice of termination and, for the avoidance of doubt, no Minimum Purchase Requirement shall apply during the Distributor 1st MHLW Lead Period or such one (1) year period and no adjustment or refund shall be made to Milestone Payment 2 already paid.
12.7 Termination for Failure to Achieve [*] Reimbursement Price.
12.7.1 Either Party may terminate this Agreement by providing written notice to other Party if Company obtains the 1st Reimbursement Approval by the Reimbursement Approval Deadline and the Reimbursement Rate in the 1st Reimbursement Approval is [*]. A Party must provide such written notice to other Party no later than ninety (90) days after the date of the 1st Reimbursement Approval and if neither Party provides such written notice within such
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
45.
ninety (90) day period, then both Parties’ rights under this Section 12.7.1 are rendered null and void. If a Party timely provides its notice of termination under this Section 12.7.1, then this Agreement shall terminate one (1) year from the date of the Party’s written notice of termination and, for the avoidance of doubt, Initial Milestone Payment 2 and Milestone Payment 2 shall not be made and no Minimum Purchase Requirement shall apply during such one (1) year period.
12.7.2 Either Party may terminate this Agreement by providing written notice to other Party if the Reimbursement Rate in the 1st Reimbursement Revision Approval is [*]. A Party must provide such written notice to other Party no later than ninety (90) days after the date of the 1st Reimbursement Revision Approval and if neither Party provides such written notice within such ninety (90) day period, then both Parties’ rights under this Section 12.7.2 are rendered null and void. If a Party timely provides its notice of termination under this Section 12.7.2, then this Agreement shall terminate one (1) year from the date of the Party’s written notice of termination and, for the avoidance of doubt, Milestone Payment 2 shall be calculated and paid as set forth in Sections 3.1.6(a) and 3.1.7, and no Minimum Purchase Requirement or True-Up Payment obligations shall apply during such one (1) year period (but shall apply with respect to prior periods).
12.8 Termination for Failure to Achieve [*] Reimbursement Price.
12.8.1 Distributor may terminate this Agreement by providing written notice to Company if, notwithstanding the exercise of its commercially reasonable efforts, Company is not able to increase or maintain the Reimbursement Rate in the 1st Reimbursement Revision Approval to an amount [*]. Distributor must provide such written notice to Company no later than ninety (90) days after the date of the 1st Reimbursement Revision Approval for such termination to be effective. If Distributor does not provide such written notice within such ninety (90) day period and also does not make the Distributor 2nd MHLW Lead Election under Section 4.1.4 within such ninety (90) day period, then Company may terminate this Agreement on written notice to Distributor. Upon a Party giving notice of termination under this Section 12.8.1, then this Agreement shall terminate one (1) year from the date of the notice of termination, Milestone Payment 2 shall be paid in accordance with Sections 3.1.5(b), 3.1.6(d) and 3.1.7, as applicable, and no Minimum Purchase Requirement or True-Up Payment obligations shall apply during such one (1) year period (but shall apply with respect to prior periods).
12.8.2 If following completion of the Distributor 2nd MHLW Lead Period, Distributor does not obtain a Reimbursement Approval with a Reimbursement Rate [*] or if during the Distributor 2nd MHLW Lead Period Distributor decides to cease its efforts to attempt to obtain an increased Reimbursement Rate, then either Party may terminate this Agreement by providing written notice to the other Party. If a Party provides its notice of termination under this Section 12.8.2, then this Agreement shall terminate one (1) year from the date of the Party’s written notice of termination and, for the avoidance of doubt, Initial Milestone Payment 2 and Milestone Payment 2 shall not be adjusted or refunded and no Minimum Purchase Requirement or True-Up Payment obligations shall apply during such one (1) year period (but shall apply with respect to prior periods).
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
46.
12.9 Termination for Failure to Achieve [*] of Initial Sales Forecast. For a period of thirty (30) days after the end of the Initial Period, Company may terminate this Agreement by providing one (1) year prior written notice to Distributor if, during the Initial Period, Distributor fails to purchase Products from Company totaling at least [*] of the Dollar value set forth in the Initial Sales Forecast. If Company exercises its right to terminate this Agreement pursuant to this Section 12.9, then Distributor shall be required to pay Company the True-Up Payment for the Initial Period, however, Distributor shall, following such notice, have no Minimum Purchase Requirement or True-Up Payment obligations for the one (1) year termination period.
12.10 Termination for Failure to Achieve [*] of Fiscal Year Forecast. For a period of thirty (30) days following the completion of each Fiscal Year, Company may terminate this Agreement by providing one (1) year prior written notice to Distributor if, during such Fiscal Year, Distributor fails to purchase Products from Company totaling [*] of the Dollar value set forth in the New Sales Forecast for such Fiscal Year. If Company exercises its right to terminate this Agreement pursuant to this Section 12.10, then Distributor shall be required to pay Company the True-Up Payment for the just completed Fiscal Year; however, Distributor shall, following such notice, have no Minimum Purchase Requirement or True-Up Payment obligations for the one (1) year termination period.
12.11 Rolling Termination Right. Company may terminate this Agreement by providing one (1) year prior written notice to Distributor in the event that if in any two (2) consecutive Fiscal Years after the Initial Period, Distributor fails to purchase Products from Company totaling [*] of the combined Dollar value set forth in the New Sales Forecasts for such two (2) consecutive Fiscal Years, provided that such notice is given within thirty (30) days after the end of the second Fiscal Year (the “Rolling Termination Right”). [However, if in either of the two (2) consecutive Fiscal Years used in determining whether the Rolling Termination Right applies, Distributor purchases Products from Company totaling [*] of the Dollar value set forth in the New Sales Forecasts for such Fiscal Year, then the Rolling Termination Right shall not apply in respect of such two (2) consecutive Fiscal Years. If Company exercises its right to terminate this Agreement pursuant to this Section 12.11, Distributor shall have no Minimum Purchase Requirement or True-Up Payment obligations for the one (1) year termination period.
12.12 Termination for Insolvency. Upon the filing of a petition in bankruptcy, insolvency, or reorganization against or by either Party, or either Party becoming subject to a composition for creditors, whether by law or agreement, or either Party going into receivership or otherwise becoming insolvent (the “Insolvent Party”), this Agreement may be terminated by the other Party by giving written notice of termination to the Insolvent Party, such termination being effective immediately upon giving of such notice. If, during the Term, in Distributor’s reasonable opinion the Company experiences financial difficulties including, without limitation, material breach of a loan agreement or default under any debt instrument or security, then upon written notice by Distributor, the Company and Distributor shall enter into good faith discussions regarding an amendment to the Agreement that would allow Distributor to exclusively continue the distribution and sale of Products in the Territory notwithstanding the financial difficulties of the Company.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
47.
12.13 Effects of Termination. Termination or expiration of this Agreement for any reason will be without prejudice to any rights that will have accrued to the benefit of a Party prior to the effective date of termination or expiration. Distributor hereby expressly and irrevocably waives any rights and claims to any compensation (for goodwill, recoupment of investment or otherwise) or indemnity, in each case resulting from the termination or non-renewal of this Agreement in accordance with its terms.
12.14 Survival. The following provisions shall survive expiration or termination of this Agreement: Sections 5.10 (to the extent set forth therein), 7.2, 7.3, 7.5, 8 (to the extent set forth in Section 8.8), 9.3, and Sections 10 through 14. If any period for such survival is set forth in the foregoing referenced sections, such section shall survive for the specified period.
13. Transition In Connection with Termination of this Agreement.
13.1 Transition. Except in the event Distributor terminates this Agreement pursuant to Sections 12.3, 12.4 or 12.12 promptly upon notice of termination or, if no notice of termination has by then been sent, beginning [*] prior to the expiration of this Agreement, Distributor and Company shall use reasonably cooperate with each other to smoothly transition maintenance, repair and other customer support functions to Company and in connection therewith, the Parties shall prepare a joint communication acceptable to both Parties to be sent to such customers regarding the transition. Upon the effective date of expiration or termination of this Agreement, Company (or its agent(s), assignee(s) or designee(s)) shall accept the regulatory, marketing, sales and service responsibilities for the Products in the Territory and Distributor shall have no such responsibilities. Such responsibilities shall include, but are not limited to, distributing, marketing, selling, shipping, billing and collecting.
13.2 Inventory. During the transition period described in Section 13.1, Distributor shall have the right to continue to purchase and sell the Product for use in the Pre-Reimbursement Approval Channel and Post-Reimbursement Approval Channel in the Territory. Company will apply all unused [*] Credits and credits provided by Company pursuant to Sections 3.1.8, 3.3.5, and 9.3.2 first against Company’s invoices for purchases made during this period. Within sixty (60) days after the effective date of expiration or termination of this Agreement, Company will repurchase from Distributor any marketable remaining Product inventory as of the effective date of expiration or termination of this Agreement at Distributor’s acquisition transfer price; provided, however, that Products are in good and saleable condition and in their original packaging. Shipment of repurchased Products will be effected by Distributor for Company’s account in accordance with Company’s instructions. No later than forty-five (45) days after receipt of the repurchased Products by Company, Company will pay Distributor in cash by wire transfer to such bank account as designated by Distributor (a) the purchase price for the repurchased Products and (b) the remaining balance of any unused [*] Credits and credits provided by Company pursuant to Sections 3.1.8, 3.3.5, or 9.3.2 as of the effective date of expiration or termination of this Agreement.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
48.
14. General Provisions.
14.1 Dispute Resolution. Except as provided in Section 14.2, each Party shall use its best efforts to resolve any dispute between them promptly and amicably and without resort to any legal process if feasible within [*] of receipt of a written notice by one Party to the other Party of the existence of such dispute. Except as provided in Section 14.2, no further action may be taken under this Section 14.1 unless and until executive officers of each of the Parties have met in good faith to discuss and settle such dispute. The foregoing requirement in this Section 14.1 shall be without prejudice to either Party’s rights, if applicable, to terminate this Agreement under Section 12.
14.2 Litigation Rights Reserved. If any dispute arises with regard to the alleged breach of Sections 5.10, 7, or 8, a Party may seek any available remedy at law or in equity from a court of competent jurisdiction.
14.3 Governing Law. This Agreement, and its formation, operation and performance shall be governed, construed, performed and enforced in accordance with the substantive laws of the State of New York, U.S.A., excluding: (a) its choice of law rules; and (b) the United Nations Convention on Contracts for the International Sale of Goods.
14.4 Arbitration. Except as set forth in Section 14.2 and this Section 14.4, all disputes, controversies or claims which may arise between the Parties hereto out of or in relation to or in connection with this Agreement, any breach hereof, including, any claim that this Agreement, or any part hereof, is invalid, illegal or otherwise voidable or void, shall be finally settled under the Rules of Arbitration of the International Chamber of Commerce (the “Rules”) by three arbitrators. Each Party shall nominate one arbitrator and the two arbitrators so selected shall nominate the third arbitrator. If the Parties’ two arbitrators cannot agree on a third arbitrator, the third arbitrator shall be appointed by the International Court of Arbitration of the International Chamber of Commerce in accordance with the Rules. The place of arbitration shall be San Francisco, California, U.S.A. The language of the arbitration shall be English. Unless the Parties otherwise agree, the arbitrators shall apply the International Bar Association Rules on the Taking of Evidence in International Commercial Arbitration. Unless the Parties otherwise agree, the arbitrators shall not have the power to appoint experts. The arbitrators shall not issue any award, grant any relief or take any action that is prohibited by or inconsistent with the provisions of this Agreement and may not, under any circumstances, award punitive or exemplary damages except to the extent permitted by Section 11.2. The award rendered by the panel of arbitrators shall be binding upon the Parties hereto and judgment on the award may be entered in any court having jurisdiction thereof. Each Party shall bear its own attorneys’ fees in connection with any arbitral proceedings and the arbitrators shall not include attorneys’ fees in any award. Notwithstanding anything to the contrary in this Section 14.4, a Party may seek injunctive relief in any court of competent jurisdiction to prevent or stop a breach of this Agreement, prevent or stop infringement or intellectual property rights or to compel arbitration under this Section 14.4.
14.5 Currency. All amounts payable under this Agreement shall be paid in U.S. Dollars, unless otherwise specifically indicated and agreed in writing by the Parties.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
49.
14.6 Accounting Matters. The Parties do not intend for the activities of the Steering Committee to be considered deliverables for financial statement purposes such as revenue recognition. Without limiting the generality of the foregoing, the Parties agree that the amounts payable by Distributor pursuant to Section 3.1.1 are fully earned as of the Effective Date and the payments payable pursuant to Sections 3.1.2 are fully earned upon the date upon which the applicable milestone is achieved.
14.7 Language. This Agreement may be translated into languages other than English. The English language version shall govern the meaning and interpretation of this Agreement.
14.8 Notices. Any notice, request, or other document to be given to a Party under this Agreement shall: (i) be in writing; (ii) hand delivered; (iii) sent by internationally-recognized express mail or courier service which provides documentation of receipt; or (iv) sent by facsimile as follows:
| If to Company: | Neuronetics, Inc. | |
| ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇ | ||
| ▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ ▇▇▇ | ||
| Attention: ▇▇▇▇▇ ▇▇▇▇▇▇▇▇, President and CEO | ||
| If to | Teijin Pharma Limited | |
| Distributor: | 2-1, ▇▇▇▇▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, | |
| ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇ | ||
| Attention: | ||
| With a copy to: | Squire Gaikokuho Kyodo Jigyo Horitsu Jimusho | |
| Ebisu Prime Square Tower, 16th Floor ▇-▇-▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇ ▇▇▇-▇▇▇▇, | ||
| ▇▇▇▇▇ | ||
| Attn: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ | ||
A Party may change its address for receiving notices, requests or other documents by giving written notice of the change to the other Party.
14.9 No Oral Modifications. This Agreement may not be modified except in writing signed by both Parties.
14.10 No Implied Waiver. The failure of one Party to require performance by the other of any provision of this Agreement will not affect the right to require performance at a later time. The waiver by one Party of a breach by the other of any provision of this Agreement shall not be a waiver of any later breach of that or any other provision hereof.
14.11 Assignment.
14.11.1 Distributor may not assign this Agreement or any rights or obligations under this Agreement, in whole or in part, to any third party without the prior written consent of Company, which consent shall not be unreasonably withheld or conditioned, and any assignment by Distributor without such consent will be null and void and of no force or legal effect. Notwithstanding the foregoing sentence, Distributor may assign this Agreement without Company’s consent to an Affiliate in the event of a merger or consolidation of Distributor with an Affiliate. No assignment shall relieve Distributor of its obligations under this Agreement.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
50.
14.11.2 Company may assign this Agreement or any right or obligation under this Agreement, in whole or in part, to an Affiliate or third party, whether by Change of Control or otherwise, so long as such Affiliate or third party is bound by operation of law to all of Company’s obligations as set forth in the Agreement and Distributor’s rights hereunder will continue as set forth herein or Company obtains the written agreement of such third party to undertake all of Company’s obligations as set forth in the Agreement and that Distributor’s rights hereunder will continue as set forth herein, and any assignment by Company without such operation of law or agreement will be null and void and of no force or legal effect. No assignment shall relieve Company of its obligations under this Agreement.
14.11.3 In the event of an assignment or transfer to an Affiliate, the assigning or transferring Party shall remain responsible (jointly and severally) with such Affiliate for the performance of such assigned or transferred obligations.
14.11.4 To the extent permitted by this Agreement, this Agreement shall be binding upon and inure to the benefit of the successors and permitted assigns of Company and Distributor. Any assignment or transfer, or attempted assignment or transfer, by either Party in violation of this Section 14.11 will be null and void and of no legal effect.
14.12 Taxes. Any taxes, levies or other duties (“Taxes”) paid or required to be withheld under the appropriate tax laws by one Party (“Withholding Party”) on account of monies payable to the other Party under this Agreement shall be deducted from the amount of monies otherwise payable to the other Party under this Agreement. The Withholding Party shall secure and send to the other Party within a reasonable period of time proof of any such Taxes paid or required to be withheld by the Withholding Party for the benefit of the other Party. The Parties shall cooperate reasonably with each other to ensure that any amounts required to be withheld by either Party are reduced in amount to the fullest extent permitted by Applicable Law. No deduction shall be made, or a reduced amount shall be deducted, if the other Party furnishes a document from the appropriate tax Governmental Authorities to the Withholding Party certifying that the payments are exempt from Taxes or subject to reduced tax rates, according to the applicable convention for the avoidance of double taxation.
14.13 Force Majeure. Neither Company nor Distributor shall be liable in damages, nor shall either of them be subject to termination of this Agreement by the other Party for any delay or default in performing any obligation under this Agreement (except payment obligations) if that delay or default is due to any cause beyond the reasonable control and without fault or negligence of that Party (“Force Majeure”); provided, however, that in order to excuse its delay or default under this Agreement, a Party shall notify the other of the occurrence or the cause, specifying the nature and particulars thereof and the expected duration thereof as soon as reasonably practical under the circumstances; and provided further that within [*] after the termination of such occurrence or cause, such Party shall give notice to the other Party specifying the date of termination thereof. All obligations of the Parties shall return to being in full force and effect upon the termination of such occurrence or cause (including, without
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
51.
limitation, any payments which became due and payable under this Agreement prior to the termination of such occurrence or cause). If an event of Force Majeure prevents either Party’s performance hereunder and continues for more than [*], the other Party may terminate this Agreement by giving written notice, however, that any amounts due shall remain payable. For the purposes of this Section 14.13, a “cause beyond the reasonable control” of a Party shall include, without limiting the generality of the phrase, any act of God, act of any government or other authority or statutory undertaking, industrial dispute, fire, explosion, accident, power failure, flood, riot or war (whether declared or undeclared), earthquake, tsunami, pandemic or outbreak of communicable disease, such as SARS.
14.14 Severability. If any provision of this Agreement is declared invalid or unenforceable by an arbitrator or court having competent jurisdiction, it is mutually agreed that this Agreement shall endure except for such provision declared invalid or unenforceable. In such event, the Parties shall consult and use their best efforts to agree upon a valid and enforceable provision which shall be a reasonable substitute for such invalid or unenforceable provision in light of the Parties’ original intent upon entry into this Agreement.
14.15 Headings and References. Section and other headings are for reference only and shall not affect the interpretation or meaning of any provision of this Agreement. Unless otherwise provided, references to Articles, Exhibits, Sections and Schedules shall be deemed references to Articles, Exhibits, Sections and Schedules of this Agreement. References to this Agreement include this Agreement as it may be modified, amended, restated or supplemented from time to time pursuant to the provisions hereof.
14.16 Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one instrument.
14.17 Entire Agreement. This Agreement, including all Schedules reference in this Agreement, contains the entire agreement between the Parties with respect to the subject matter hereof and supersedes all previous agreements and understandings, whether written or oral, between the Parties regarding the subject matter hereof.
14.18 Terms Generally. Unless the context of this Agreement requires otherwise, words importing the singular number shall include the plural and vice versa. Words importing the masculine gender shall include the feminine. In this Agreement, references to: (a) any statutory or regulatory provisions shall include such provisions as from time to time amended, whether before or after the date hereof, and shall further include all statutory or regulatory instruments or orders from time to time made pursuant thereto; and (b) any document or agreement shall include such document or agreement as from time to time amended, supplemented or replaced, whether before or after the date hereof. The words “include”, “includes” and “including” shall be deemed to be followed by the phrase “without limitation.” Unless otherwise specified, all of the terms in this Agreement that relate to accounting matters shall be interpreted in accordance with generally accepted accounting principles in effect in the United States of America at the time of such interpretation.
(signature page follows)
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
52.
IN WITNESS WHEREOF, the Parties have signed this Agreement as of the date first set forth above.
| COMPANY: | ||
| NEURONETICS, INC. | ||
| By: | /s/ ▇▇▇▇▇ ▇▇▇▇▇▇▇▇ | |
| Name: ▇▇▇▇▇ ▇▇▇▇▇▇▇▇ | ||
| Title: President and CEO | ||
| DISTRIBUTOR: | ||
| TEIJIN PHARMA LIMITED | ||
| By: | /s/ Akihisa Nabeshima | |
| Name: Akihisa Nabeshima | ||
| Title: President | ||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
.
SCHEDULE A
NEUROSTAR STARTER PACKAGE
| NeuroStar Starter |
||||||||
| Catalog Number |
Item Number |
Starter Kit |
Description |
US$ transfer | ||||
| [*] | [*] | [*] | [*] | |||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
| [*] | [*] | [*] | ||||||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
.
SCHEDULE B
Distributor Initial Sales Forecast
| Time Period |
NeuroStar TMS Therapy Systems |
Sen Star Treatment Links / Treatment Sessions | ||
| From [*] assuming each period is a full calendar year. If the period from [*] is less than a full calendar year, then amounts set forth I the two adjacent columns shall be prorated for such period. |
[*] | [*] | ||
| First full Fiscal Year after the end of the Fiscal Year [*] |
[*] | [*] | ||
| Second full Fiscal Year after the end of the Fiscal Year [*] |
Total Dollar value of sales forecast for the Initial Period=
Subtotal of Dollar value of NeuroStar TMS Therapy Systems purchased from Company during the Initial Period (calculated using transfer price determined in accordance with Section 3.5 of the Agreement):
+
Subtotal of Dollar value of SenStar Treatment Links and Treatment Sessions purchased from Company during the Initial Period: (Subtotal of treatment sessions from above chart) x transfer prices determined in accordance with Section 3.5 of the Agreement.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE C
Training and Support Programs
Sales Training Program
Appropriate Company staff shall travel at the expense of Distributor to a location of Distributor’s choice and provide sales training sufficient to make Distributor’s staff skilled in the sales of Products.
Clinical Training Program
Distributor staff to travel at their sole expense to a United States location determined by Company to receive both didactic and hands on training. Expected duration of training is two (2) weeks.
Technical Training Program
Distributor staff to travel at their sole expense to a United States location determined by Company to receive both didactic and hands on training. Expected duration of training is one (1) week. Advanced “train the trainer” training can be provided at Distributor’s expense to train Distributor personnel to conduct future distributor trainings.
Additional Company Required Training
Additional sales, clinical, regulatory, compliance and quality or technical training may be required by Company from time to time. Attendance is mandatory for appropriate Distributor staff and is at Distributor’s expense. Most trainings of this type can be conducted via webinar.
Distributor Requested Training and Support
Company shall make additional training and support available at Distributor’s request.
| Item Number |
Training and Support Type |
Cost/Day | ||
| Sales/Marketing | [*] | |||
| XXX |
Clinical Training and Support (3 day minimum) | [*] | ||
| XXX |
Technical Service Training and Support: This may include installation, repair and/or training.** (3 day minimum) |
[*] |
| * | Expenses include economy class airfare and the costs for hotel, meals, and local transportation but not to exceed [*] |
| ** | Parts are not included. |
Pricing after the Fixed Transfer Price Period is subject to mutual agreement of the Parties.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE D
Company Trademarks
English Language Trademarks Registered in Japan
| ▇▇▇▇ |
Registration NO./Date |
App. No. /Date |
Goods/Classes |
Status | ||||
| NEURONETICS |
5535065 11/9/2012 |
042942 5/29/2012 |
Medical, magnetic neuromodulators for the treatment of central nervous system disorders, psychiatric and neurological disorders in International Class 10 | Registered; Renewal due: 11/9/2022 | ||||
| NEUROSTAR |
5572813 04/05/2013 |
042943 5/29/2012 |
Transcranial magnetic stimulation devices consisting of a stimulator and a patient positioning system, namely, a chair and headset in International Class 10 | Registered; Renewal due: 4/05/2023 | ||||
| NEUROSTAR & Design |
5572814 4/05/2013 |
042944 05/29/2012 |
Transcranial magnetic stimulation devices consisting of a stimulator and a patient positioning system, namely, a chair and headset in International Class 10. | Registered; Renewal due: 04/05/2023 | ||||
| NEUROSTAR TMS THERAPY |
5572815 04/05/2013 |
042945 05/29/2012 |
Transcranial magnetic stimulation devices consisting of a stimulator and a patient positioning system, namely, a chair and headset in International Class 10 | Registered; Renewal due: 04/05/2023 | ||||
| SENSTAR |
5572816 04/05/2013 |
042946 05/29/2012 |
Medical devices, namely, a disposable patient interface for use with transcranial magnetic stimulation devices in International Class 10 | Registered; Renewal 04/05/2023 | ||||
| TMS TRAKSTAR |
5572817 | 042947 | Computer application software for use with transcranial magnetic stimulation devices, namely, software for use in database management in International Class 9 | Registered; Renewal 04/05/2023 | ||||
| NEUROSTAR XPLOR |
5631472 | 2012 97829 |
Computer software for use with transcranial magnetic stimulation devices in International Class 9 | Registered; Renewal due: 11/22/2013 |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Trademarks To Be Registered in English and Katakana in Japan
| NEURONETICS (IN KATAKANA CHARACTERS) | Medical, magnetic neuromodulators for the treatment of central nervous system disorders, psychiatric, and neurological disorders in Class 10.; • Medical research pertaining to the treatment and diagnosis of central nervous system disorders in Class 42.• Medical evaluation services pertaining to the treatment and diagnosis of central nervous system disorders Class 44. | |
| NEUROSTAR (IN KATAKANA CHARACTERS) |
Transcranial magnetic stimulation devices consisting of a stimulator and a patient positioning system, namely, a chair and headset in Class 10. | |
| TMS THERAPY |
Transcranial magnetic stimulation devices consisting of a stimulator and a patient positioning system, namely, a chair and headset in International Class 10. | |
| NEUROSTAR XPLOR (IN KATAKANA CHARACTERS) |
Computer software for use with transcranial magnetic stimulation devices, namely, software for use in database management in Class 9; • Transcranial magnetic stimulation devices consisting of a stimulator and a patient positioning system, namely, a chair and headset in Class 10. | |
| TMS TRAKSTAR (IN KATAKANA CHARACTERS) |
Computer application software for use with transcranial magnetic stimulation devices, namely, software for use in database management in Class 9. | |
| SENSTAR (IN KATAKANA CHARACTERS) |
Medical devices, namely, a disposable patient interface for use with transcranial magnetic stimulation devices in International Class 10. | |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
| MT ASSIST (IN KATAKANA CHARACTERS) |
Computer software for aiding in the computation of the optimal stimulation level for the treatment of central nervous system disorders, including psychiatric and neurologic disorders computer software for aiding in the computation of the optimal stimulation level for the treatment of central nervous system disorders, including psychiatric and neurologic disorders in Class 10. | |
| PRECISION PULSE TMS (IN KATAKANA CHARACTERS) |
Electric coils, electric circuits and electric control consoles for use with transcranial magnetic stimulation devices in Class 9. | |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE E
Visual Inspection Procedure
| 1) | At the time of installation, a visual inspection under normal room lighting and areas where parts and products are viewed under normal use is conducted to ensure that no damage occurred to the NeuroStar Starter Package during shipment and transportation. |
| 2) | Crates must be inspected when they are received and any damage identified must be noted at that time in accordance with the carrier’s damage reporting procedure. Crate damage at time of receipt by Distributor is considered in-transit damage and is not covered by these instructions. Distributor may make a warranty claim for NeuroStar Product damaged in-transit. |
| 3) | The person witnessing or performing the visual inspection at the time of installation must be a trained NeuroStar Field Service Engineer. |
| 4) | The treatment chair should be inspected for the following: |
| a) | Cushion for any rips, tears or holes. |
| b) | Covers for scratches, cracks, or breakage. |
| c) | Frame for bends or breakage. |
| d) | Adjustable feet to ensure they are not bent or crooked. |
| e) | Missing or loose hardware. |
| 5) | The console should be inspected for the following: |
| a) | Covers for scratches, cracks, or breakage. |
| b) | Wheels to ensure they are not bent or crooked. |
| c) | Missing or loose hardware. |
| d) | Display screen to ensure no damage. |
| 6) | Head support |
| a) | Cushion for any rips, tears, or holes. |
| b) | Covers for scratches, cracks, or breakage. |
| c) | Missing or loose hardware. |
| 7) | A NeuroStar Starter Package which fails a prescribed visual inspection must not be put into service until the issue is resolved. |
| 8) | If an issue is identified as part of the visual inspection conducted at time of installation, the NeuroStar Field Service Engineer witnessing or performing inspection should attempt to resolve the issue according to the Distributor Service Manual and in accordance with the individual’s FSE Training. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE F
Return of Products
Any product for which Distributor receives a technical complaint and for which a repair 1s determined to be required, Distributor will replace the part using a product Field Replacement Unit.
Return of Products requires that Distributor complete and print a Technical Event Report in such format as Company shall notify to Distributor from time to time and attach it to the Field Replacement Unit. Distributor must also email the completed Technical Event Report to Company at such email address as notified by Company to Distributor, such email being as of the Effective Date: ▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇.▇▇▇. Distributor ships Field Replacement Units to be returned to Company at Distributor’s expense. Distributor will ship Products as soon as practicable where a potential safety issue is asserted. Otherwise, Distributor will ship items for return so they are received by the Company within 30 days after Distributor receives the items from their customer. Timely shipment is required for Company to investigate and address complaints within required timeframes.
Company Technical Support logs emailed information from the Technical Event Form into Company’s complaint database and processes the complaint according to Company’s complaints procedure. Returned items are processed by Company according to Company’s non-conforming material (NMR) procedure.
Replacement parts are sent at Company’s expense to Distributor for parts which are under warranty. The cost of repairs and shipping to Distributor for Product not under warranty is the responsibility of Distributor.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE G
| Quarterly Update |
Strategic Growth Drivers Full Year 2017
| Initiative / Program |
Implementation date | |||
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 |
[*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE H
Anti-Bribery Due Diligence Documentation
(see attached)
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
Anti-Bribery Compliance Certification
Pursuant to the Distribution Agreement between Neuronetics, Inc., and Teijin Pharma Limited, dated 10 October I hereby certify:
[*]
[*]
[*]
COMPANY NAME: Teijin Pharma Limited
PRINT NAME: ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇
TITLE: General Manager, Home Healthcare New Business Development Division
SIGNATURE: /s/ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇
DATE: October 10, 2017
▇▇-▇▇▇▇▇-▇▇▇ Rev A
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
Distributor Qualification Form
You are requested to complete this form and return it to your Neuronetics’ Business Development representative. To ensure accurate information is provided, each section should be complete by or reviewed with your companies’ department representatives.
[*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE I
Minimum Terms and Conditions of Sale
| 1. | The System [this term will be defined in Distributor’s agreement with its customer] is sold to the customer with the understanding that the operation of the System must be undertaken only in a manner that is compliant with the NeuroStar TMS Therapy System User Manual. The System can only be used in accordance with the laws of Japan. |
| 2. | Use of the System by a customer is permitted only by users authorized in accordance with the laws of Japan. Such operators are referred to as “Authorized Users.” |
| 3. | The customer shall be responsible to ensure that all Authorized Users have the requisite training and skill required to use the System as defined by all applicable regulatory and medical authorities in Japan. Customer will, at all times, ensure that it and its employees and agents and all Authorized Users |
| 4. | Distributor grants the customer and its Affiliates that will use the Products a limited, nonexclusive, non-transferable (except as set forth in Item IO below) and non-sublicensable (except as expressly provided in Item 6 below) right and license to operate and use the Software (along with any written or electronic documentation provided with the Software or System) solely in conjunction with the operation and use of the System. The customer obtains no right, title or interest in or to the Software, except for the limited license so granted, and Distributor and its licensors reserve all rights not expressly granted. |
| 5. | The customer and its Affiliates may use the Software only in connection with the use and operation of the System in accordance with Neuronetics, Inc.’s instructions as conveyed by Distributor to customer or the documentation. Except as expressly provided in Item 6 below, customer may not loan, rent, lease, license or otherwise transfer to any other person, or host on behalf of any other person, the Software, and may not copy, modify, remove, disassemble, create derivative works from or tamper with the Software except to an Affiliate that will use the Products. Any attempted transfer or use of the Software without the prior consent of Distributor, except as expressly provided in Item 6 or 10 below, will void the license. |
| 6. | A customer that is a leasing or finance company that has acquired a System or Systems for the purpose of leasing such System(s) to a third party shall be permitted to sublicense the Software licenses granted hereunder to the lessee(s) of such System(s) provided that all such lessees agree in writing to be bound by these minimum end-user license agreement terms. Any such sublicense shall terminate upon the expiration or termination of the underlying lease agreement. |
| 7. | Customer may use the TrakStar Software and the associated documentation only for its or its Affiliates own internal use on the computer system provided with the NeuroStar TMS Therapy System or a replacement computer as authorized and installed by Distributor. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
.
| 8. | Customer may not copy or otherwise reproduce the Software or documentation. Except as expressly permitted under applicable law, customer may not decompile, reverse engineer or disassemble the Software in an attempt to derive or use the source code therefrom. |
| 9. | Software may include Redistributable Code, which is the property of Neuronetics, Inc.’s licensors, and protected under United States and international copyright, trade secret or other proprietary rights laws, as well as international treaties. Distributor grants to customer and its Affiliates a limited, non-exclusive, non-sublicensable and nontransferable right and sub-license to use and display the Redistributable Code solely in connection with the authorized use of the Software in connection with the operation of the System and in conformance with the other minimum end-user license agreement terms. Except as expressly permitted under applicable law, customer and its Affiliates may not reproduce, redistribute, decompile, reverse engineer or disassemble the Redistributable Code and may not dis-integrate the Redistributable Code from the Software. |
| 10. | Customer may only transfer the Software and the Redistributable Code as part of a sale or transfer of the System to a third party and the third party must agree to the minimum end-user license terms as a condition to such transfer, provided however that customer may transfer the Software and the Redistributable Code to an Affiliate along with the System. |
| 11. | DISTRIBUTOR AND NEURONETJCS SHALL NOT BE LIABLE FOR INCIDENTAL, CONSEQUENTIAL, INDIRECT OR SPECIAL DAMAGES OF ANY KIND, INCLUDING BUT NOT LIMITED TO DAMAGES FOR LOST REVENUE OR LOST PROFITS, LOSS OF DATA, LITJGATJON EXPENSE, DAMAGE TO REPUTATION, LOSS OF BUSINESS OR ANY OTHER FINANCIAL LOSS ARISING OUT OF OR IN CONNECTION WITH THE SOFTWARE. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE J
NeuroStar Treatment Session Transfer Price Table
| Reimbursement Rate/Treatment Session in JPY |
NeuroStar Treatment Sessions in USD | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] | |
| [*] |
[*] |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
1. If the reimbursement price falls between two reimbursement prices set forth in above, then the transfer price will be determined in accordance with the following formular:
[*]
2. If the reimbursement price determined by MHLW is lower than the lowest reimbursement price set forth in the above table, then [*] reimbursement price determined by MHLW is higher than the highest reimbursement price set forth in the above table, then [*]
3. If the reimbursement rate determined by MHL W is paid as a single payment for a complete course of treatment, this reimbursed amount will be divided by the number of treatments foreseen as a course of treatment by MHLW or 30, whichever is smaller, to determine the Reimbursement Rate/Treatment Session used in the table above in determining the transfer price. If the reimbursement varies by treatment session, the average reimbursement over a treatment course of 30 treatment sessions will be used for the Reimbursement Rate/Treatment Session in the table above in determining the transfer price. If any other reimbursement scenario is set by MHLW, the average reimbursement over a 30 treatment course will be used for the Reimbursement Rate/Treatment Session in the table above in determining the transfer price.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE K
NNI Technical Support
See Attached
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||
| Page 1 of 10 | UNCONTROLLED IF PRINTED | |
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
| 1. | PURPOSE |
| 1.1 | This Distributor Quality Plan (this “Plan”) specifies processes, procedures and resources for distribution, service and defect management of Products (as defined in Section 4) that are clinically available in the Territory (as defined in Section 4). |
| 1.2 | This Plan is intended to ensure compliance with Applicable Laws (as defined in Section 4) including those cited in Section 3.0 Reference Documents. |
| 2. | SCOPE |
| 2.1 | This procedure applies to processes and procedures conducted for Products distributed in the Territory by Distributor (as defined in Section 4). |
| 2.2 | This procedure applies to employees of Company (as defined in Section 4) and Distributor in the Territory. |
| 3. | REFERENCE DOCUMENTS |
| 3.1. | 21 CFR Part 820, Quality System Regulations |
| 3.2. | ISO 13485:2003, Medical Devices - Quality Management Systems |
| 3.3. | MHLW Pharmaceuticals and Medical Device Act (Japan) |
| 3.4. | MHLW Ministerial Ordinance No. 169, 2004, Good Manufacturing Practices (Japan) |
| 3.5. | ▇▇-▇▇▇▇▇-▇▇▇, Quality Manual |
| 3.6. | ▇▇-▇▇▇▇▇-▇▇▇, Work Instruction- Medical Event Reporting to Neuronetics |
| 3.7. | ▇▇-▇▇▇▇▇-▇▇▇, Medical Event Form |
| 3.8. | ▇▇-▇▇▇▇▇-▇▇▇, OUS Technical Event Recording procedure |
| 3.9. | ▇▇-▇▇▇▇▇-▇▇▇, Distributor Training |
| 3.10. | ▇▇-▇▇▇▇▇-▇▇▇, NeuroStar OUS Installation Record |
| 4. | DEFINITIONS |
| 4.1. | “Agreement” means the Distribution Agreement between Company and Distributor as amended from time to time. |
| 4.2. | “Applicable Laws” means all Laws, including cGMP, including, without limitation, any rules, regulations, guidelines or other requirements of the FDA and any other Governmental Authority with respect to the manufacture of the Products and rules, regulations, guidelines or other requirements of MHLW applicable to the promotion, marketing, distribution and sale of the Products in the Territory. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||
| Page 2 of 10 | UNCONTROLLED IF PRINTED | |
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
| 4.3. | “Business Day” means a day (other than Saturday or Sunday) on which banks are open for business in Tokyo, Japan, and in New York, New York. |
| 4.4. | “cGMP” means the current good manufacturing practices applicable to the manufacture of the Products as defined in the U.S. Current Good Manufacturing Practices, 21 C.F.R. Part 820, and the equivalent Laws in the Territory, each as may be amended and applicable from time to time. |
| 4.5. | “Company’’ means Neuronetics, Inc., a Delaware corporation having its principal offices at ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇, ▇▇▇. |
| 4.6. | “Distributor” means Teijin Pharma Limited, a Japanese company having its principal offices at 2-1, ▇▇▇▇▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇. |
| 4.7. | “DMAH” means the agent approved by the MHLW to act as the marketing authorization holder in accordance with and as defined in the Law on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics. |
| 4.8. | “Event of Special Interest” means any of the following events regardless of its causal relationship to use of the Products: (a) seizure: patient experiences a seizure; (b) pregnancy: a patient receiving therapy with the Products is pregnant or becomes pregnant; (c) worsening illness or suicide attempt/suicidal ideation: a patient is hospitalized due to worsening illness, makes a suicide attempt or the occurrence of a completed suicide; or (d) Device-Device Co-Administration: lf TMS Therapy is administered in a patient with an implanted medical device and a device-device interaction is suspected or occurs. |
| 4.9. | “FDA” means the United States Food and Drug Administration and any successor thereto. |
| 4.10. | “Governmental Authority’’ means any multinational, national, federal, prefectural, state, local, municipal or other governmental authority of any nature (including any governmental division, subdivision, department, agency, bureau, branch, office, commission, council, court or other tribunal), in each case, having jurisdiction over the applicable subject matter. |
| 4.11. | “Laws” means all laws, statutes, rules, regulations, directives, decisions and ordinances of any Governmental Authority. |
| 4.12. | “Medical Event” means any event that requires clinical attention or intervention and is determined by the evaluating clinician to be possibly related, probably related, or definitely related to the use of the Products or for which exact causal relationship cannot be determined at time of event or report. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||
| Page 3 of 10 | UNCONTROLLED IF PRINTED | |
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
| 4.13. | “MHLW” means the Japanese Ministry of Health, Labour and Welfare and any successor thereto. |
| 4.14. | “PMDA” means the Japanese Pharmaceuticals and Medical Devices Agency and any successor thereto. |
| 4.15. | “Products” means the products the Distributor distributes in the Territory pursuant to the Agreement. |
| 4.16. | “Territory” means Japan. |
| 5. | RESPONSIBILITIES |
| 5.1. | Company shall: |
| • | Be responsible for the manufacture, shipping, exportation and importation of the Products for use in the Territory in compliance with all Applicable Laws including the standards and regulations as cited in Section 3.0. |
| • | Be responsible for ensuring that export and import documentation are obtained for the Products leaving the US and entering the Territory. |
| • | Obtain a quality agreement with the DMAH and operate according to the requirements of said agreement. |
| • | Provide and/or review quality and regulatory information relative to the Products distributed in the Territory in a timeframe sufficient for timely action in accordance with Company’s quality management system and Applicable Laws. |
| • | Provide advance notification to DMAH of changes to the Product in sufficient time to allow for review and approval by PDMA, if required. Periodically provide relevant post-market surveillance information to Distributor pertaining to device performance. |
| • | Provide annual distributor training per procedure ▇▇-▇▇▇▇▇-▇▇▇, Distributor Training. |
| • | Consult with DMAH for interactions with MHLW, PDMA and other relevant Governmental Authorities in the Territory related to use of the Products in the Territory. |
| • | Perform its obligations set forth in the Agreement in compliance with Applicable Laws. |
| • | Document and communicate to DMAH in a timely manner information relevant to the safe and effective operation of the Products in the Territory including product defects, technical event complaints, medical event complaints, and any quality complaints related to the Products. |
| • | Communicate to Distributor in a timely manner updated instructions for the safe and effective operation of the Products in the Territory. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||
| Page 4 of 10 | UNCONTROLLED IF PRINTED | |
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
| 5.2. | Distributor shall: |
| • | Ship, store, distribute, install and service the Products in the Territory in accordance with the Agreement. |
| • | Perform its obligations set forth in the Agreement in compliance with Applicable Laws. |
| • | Perform its activities in the Territory with respect to the Products in accordance with Applicable Laws. |
| • | Track and document the Products at installation, servicing and repair and retain and maintain such documentation in accordance with Sections 5.3.2 and 5.10.2. |
| • | Document and communicate to Company and DMAH in a timely manner information relevant to the safe and effective operation of the Products in the Territory including product defects, technical event complaints, medical event complaints, and any quality complaints received from Distributor’s customers related to the Products. |
| • | Respond promptly to all inquiries from Distributor’s customers, including but not limited to, customer feedback and service requests. |
| • | Collaborate with Company and the DMAH in the investigation of complaints, adverse event reports and technical events and determination of closure or corrective actions, including DMAH’s notification of Governmental Authorities |
| • | Obtain a quality agreement with the with the DMAH and operate according to the requirements of said agreement. |
| 5.3. | Control of Records |
| 5.3.1. | Company shall ensure that applicable data and documents are housed in a secured and access-controlled SharePoint site that is dedicated to information and records shared between Company and Distributor. |
| 5.3.2. | Distributor shall create records for identification and traceability of the Products distributed by Distributor in the Territory and maintain such records for the longer of the timeframe required by Applicable Laws or 15 years. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||
| Page 5 of 10 | UNCONTROLLED IF PRINTED | |
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
| 5.4. | Infrastructure and Work Environment |
| 5.4.1. | Distributor shall use a storage environment which prevents damage and maintains the shelf and operating life of the Products while the Products are stored at facilities under Distributor’s control in the Territory prior to delivery to Distributor’s customers. |
| 5.4.2 | Distributor shall or shall ensure that Distributor’s subcontractors follow the installation procedures for the Products at customer sites as set forth in documentation provided by Company to ensure a safe operational environment for clinical use. |
| 5.5. | Service and Technical Events and Medical Events |
| 5.5.1. | Distributor and Company will follow the processes and procedures set forth in Appendices A and B, as applicable, in relation to service and technical events and medical events related to the Products reported by Distributor’s customers in the Territory. |
| 5.6. | Recall and Advisory Notices |
| 5.6.1. | DMAH in collaboration with Company shall communicate with Governmental Authorities in the Territory regarding any recalls, advisory notices and other regulatory actions. |
| 5.6.2. | Distributor shall assist in any regulatory request or action or advisory notice or recall by providing directly to Company and DMAH all customer, traceability and inventory information in its possession or control that is needed to assess and respond to such request or action, provide such advisory notice or perform such recall. |
| 5.6.3 | Company and the DMAH have sole authority to issue a recall or require corrective action with respect to Products in the Territory including those required by Applicable Laws or a Governmental Authority in the Territory. |
| 5.6.4 | Company and DMAH are responsible to plan and lead the recall with input from Distributor, and Distributor shall be responsible for executing the plan in the Territory including distributing communications to customers, performing all field activities in the Territory creating and maintaining required records, including inventory records and, if necessary, the physical return of Products from the Territory. |
| 5.6.5 | Company and DMAH with the assistance of Distributor are responsible for preparing advisory notices and other communications to customers. |
| 5.6.6 | Company shall communicate to DMAH and Distributor reportable regulatory actions initiated outside of the Territory that are applicable to the Products distributed in the Territory including recalls and advisory notices and coordinating any required actions with DMAH and Distributor. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||
| Page 6 of 10 | UNCONTROLLED IF PRINTED | |
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
| 5.7. | Customer Feedback |
| 5.7.l. | Distributor shall collaborate with Company and DMAH directly in matters of vigilance and post-market surveillance. |
| 5.7.2. | Distributor shall report product complaints to Company and DMAH in writing within ten (10) Business Days from the date of receipt of the product complaint. |
| 5.7.3. | Distributor shall report urgent issues including Medical Events and Events of Special Interest within two (2) business days after Distributor receives a report of any such issue from its customer or a physician using a NeuroStar Product in the Territory sold by Distributor and, to the extent applicable, in accordance with Appendix A. |
| 5.8. | Design and Development |
| 5.8.1. | Company shall provide Distributor with six (6) months prior written notice of any changes (i) the form, fit or function of any of the Products or (ii) any part, process or manufacturer of the Products, which change will require the approval of any Governmental Authority in the Territory or would be expected to have a material impact on the quality control processes or the quality of the Products. |
| 5.9. | Product Inspection |
| 5.9.1. | Company shall ensure that appropriate inspection certification(s) for the Products are kept on file at Company or at DMAH, as applicable. |
| 5.9.2. | Company shall provide certificates of conformance that verify that all Products meet all device specifications. |
| 5.9.3. | Company shall ensure that DMAH receives and inspects the Products in accordance with the inspection procedure agreed between Company and DMAH. |
| 5.9.4. | Distributor shall receive delivery of Products from the carrier after DMAH releases the Products in the Territory. |
| 5.9.5. | Distributor is responsible for installing the Products at the customer site and performing all inspection and other procedures required by NeuroStar OUS Installation Record (25-11512-000) and providing the completed NeuroStar OUS Installation Record to Company. |
| 5.10. | Identification and Traceability |
| 5.10.1. | Control numbers or unique device identifiers are used by Company for tracking inventory location, distribution, installation, and, as necessary, shelf life of the Products. The control numbers are necessary in the investigation of complaints and/or recall activities. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||
| Page 7 of 10 | UNCONTROLLED IF PRINTED | |
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
| 5.10.2. | Distributor is responsible for maintaining ID and traceability for the NeuroStar TMS Therapy System, SenStar Treatment Link, SenStar Connect and serialized components (Field Replaceable Units) that are stored and used in service and distribution in the Territory. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||
| Page 8 of 10 | UNCONTROLLED IF PRINTED | |
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
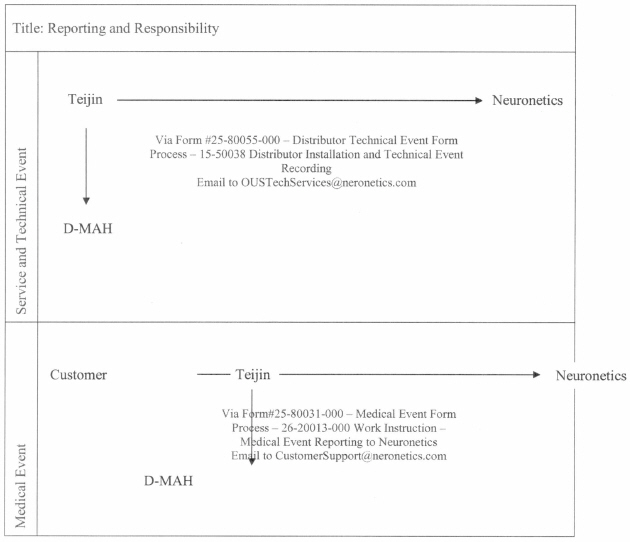
Appendix A
CONFIDENTIAL & PROPRIETARY INFORMATION OF NEURONETICS, INC.
NOT TO BE REPRODUCED OR USED IN ANY MANNER OTHER THAN WITH THE EXPRESS WRITTEN PERMISSION OF NEURONETICS INC.
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||||
| Page 9 of 10 | UNCONTROLLED IF PRINTED | |||
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
Appendix B
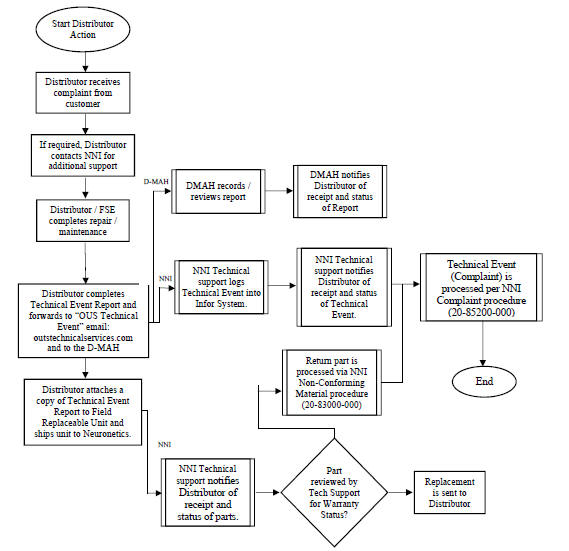
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
| Standard Operating Procedure | ||||
| Page 10 of 10 | UNCONTROLLED IF PRINTED | |||
| ▇▇-▇▇▇▇▇-▇▇▇ Rev C | Distributor Quality Plan, Teijin |
REVISION INFORMATION
| Rev |
CN No |
Written/Revised By |
Date |
Revision Description | ||||
| A | 4673 | ▇▇▇▇ ▇▇▇▇▇▇▇▇▇ | 08SEP2016 | Initial Release | ||||
| B | 5193 | ▇▇▇▇▇ Heart | 04OCT2017 | Flowchart updated to include notification to distributor of receipt of technical event report and parts. Updated to include DMAH in reporting of medical events | ||||
| C | 5198 | ▇▇▇▇▇ Heart (▇▇▇ ▇▇▇▇▇▇) | 05OCT2017 | Synchronization of language to reflect contract information. | ||||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
Page 1 of 5
| Document Name: | XX-XXXXX-XXX Japanese Distributor Installation and Technical Event Recording | Effective Date: | 1-Aug-17 | |||
| Revision Letter: | A | Last Review Date: | 1-Aug-17 |
PURPOSE
The purpose of this document is to define the process by which Distributor completes installation activities and records and communicates technical events for the Products.
SCOPE
| • | This procedure is applicable as a part of Distributor’s responsibilities for product quality assurance as described in the Distributor Quality Plan. |
| • | This procedure also covers Distributor’s responsibility to complete installation records for the equipment it installs. |
| • | Neuronetics Technical Service provides support to Distributor including training, documentation and advanced trouble shooting. |
3.0 REFERENCE DOCUMENTS
| 3.1 | ▇▇-▇▇▇▇▇-▇▇▇ Distributor Quality Plan |
| 3.2 | ▇▇-▇▇▇▇▇-▇▇▇ Distributor Technical Event Form |
| 3.3 | ▇▇-▇▇▇▇▇-▇▇▇ NeuroStar OUS Installation Record |
| 3.4 | ▇▇-▇▇▇▇▇-▇▇▇ Customer Complaint |
| 3.5 | ▇▇-▇▇▇▇▇-▇▇▇ Non-conforming material procedure |
| 3.6 | ▇▇-▇▇▇▇▇-▇▇▇ NeuroStar Distributor Service Manual |
4.0 DEFINITIONS
| 4.1 | “Agreement” means the Distribution Agreement between Company and Distributor as amended from time to time. |
| 4.2 | “Company” means Neuronetics, Inc., a Delaware corporation having its principal offices at ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇, ▇▇▇. |
| 4.3 | “DMAH” means the agent approved by the MHLW to act as the marketing authorization holder in accordance with and as defined in the Law on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics. |
| 4.4 | “Distributor’ means Teijin Pharma limited, a Japanese company having its principal offices at 2-1, ▇▇▇▇▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇. |
| 4.5 | “DSO” or “Distributor Service Organization” means the group within the Distributor that is responsible for installation and field service of the Products in the Territory. The DSO may be Distributor’s Technical Support Company (as defined in the Agreement). |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
Page 2 of 5
| Document Name: | XX-XXXXX-XXX Japanese Distributor Installation and Technical Event Recording | Effective Date: | 1-Aug-17 | |||
| Revision Letter: | A | Last Review Date: | 1-Aug-17 |
| 4.6 | “NTS” or “Neuronetics Technical Service” means a group within Neuronetics Field Service that provides third level support. Third level support is defined as advanced field service troubleshooting, Field Service Engineer training, service documentation creation and service system development. |
| 4.7 | “Out of Box Failure” is as defined in the Agreement. |
| 4.8 | “Products” means the products the Distributor distributes in the Territory pursuant to the Agreement. |
| 4.9 | “Territory” means Japan. |
5.0 RESPONSIBILITIES
| 5.1 | Director of Field Service - Ensures that Distributor’s field service/technical support organizations are trained on equipment install procedures and technical event reporting process. |
| 5.2 | DSO is responsible for completing technical event report(s) and installation record(s) and forwarding to Neuronetics technical support via “OUS Technical Support” email and to the DMAH. |
6.0 PROCEDURE
| 6.1 | Distributor Service Organization (Installation Activity- See Attachment B for Flow Chart) |
| 6.1.1 | DSO completes installation activity and fills out NeuroStar OUS Installation Record (25-11512-000). |
| 6.1.2 | Installation record is provided to Neuronetics technical support via email ▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ or equivalent electronic transfer as well as to the DMAH. |
| 6.1.3 | An Out of Box Failure during the installation process is processed per Section 6.2 of this document and Sections 9.3.2 and 9.3.5 of the Agreement. |
| 6.2 | Distributor Service Organization (Technical Event Recording—see Attachment A for Flow Chart) |
| 6.2.1 | DSO receives technical complaint from NeuroStar customer. DSO may contact Neuronetics technical support if required. |
| 6.2.2 | DSO services product and completes the repair. DSO completes Technical Event Report (25-80055-000), and emails completed form to Neuronetics via “email (▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇.▇▇▇) as well as to the DMAH. Neuronetics Technical Support logs information from Technical Event Report into the complaint database (lnfor system). |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
NEURONETICS
Page 3 of 5
| Document Name: | XX-XXXXX-XXX Japanese Distributor Installation and Technical Event Recording | Effective Date: | 1-Aug-17 | |||
| Revision Letter: | A | Last Review Date: | 1-Aug-17 |
| 6.2.3 | DSO prints completed technical event report (25-80055-000) and attaches to the field replaceable unit. |
| 6.2.4 | DSO ships defective Field Replaceable Unit to Neuronetics. |
| 6.2.5 | Neuronetics receives Field Replaceable Unit which is processed via Neuronetics Complaints procedure and non-conforming material (NMR) process. |
| 6.2.6 | Replacement is sent by NNI for parts which are under warranty. |
| 6.2.7 | For parts that are not under warranty, the DSO places an order for a replacement part. |
| 6.2.8 | Neuronetics will report in writing appropriate information to Distributor concerning root cause analysis, likelihood of re-occurrence, impact on Product performance, workarounds and planned fixes, as applicable. Such reporting will generally be completed within two to four weeks after Neuronetics’ receipt of the technical event report and defective Field Replaceable Unit. |
7.0 AVAILABILITY OF NEURONETICS TECHNICAL SUPPORT
| 7.1 | Neuronetics provides live technical support by phone and email from 8AM ET (USA) to 6PM ET (USA). An answering service answers the technical support phone number after hours. Neuronetics generally responds to technical support requests within 24 hours after receipt other excluding weekend and holidays. |
8.0 QUALITY RECORDS
| 8.1 | Neurostar OUS Installation Record (25-11512-000) |
| 8.2 | OUS Technical Event (25-80055-000) |
9.0 ATTACHMENTS
| 9.1 | Attachment A- Process flow chart |
| 9.2 | Attachment B- Install flow chart |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 4 of 5
| Document Name: | XX-XXXXX-XXX Japanese Distributor Installation and Technical Event Recording | Effective Date: | [*] | |||
| Revision Letter: | A | Last Review Date: | [*] |
Attachment A – Technical Event Process
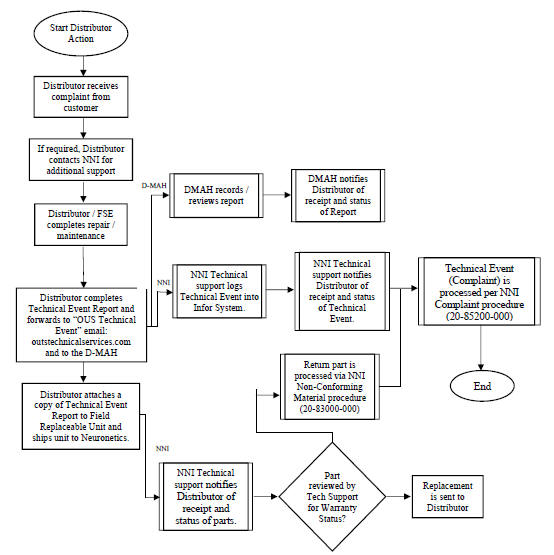
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Page 5 of 5
| Document Name: | XX-XXXXX-XXX Japanese Distributor Installation and Technical Event Recording | Effective Date: | [*] | |||
| Revision Letter: | A | Last Review Date: | [*] |
Attachment B – Technical Event Process

[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
| NEURONETICS | NeuroStar – OUS Installation Record |
NeuroStar TMS Therapy System
Installation / Operation Qualification
| Note: | Please refer to the NeuroStar Distributor Service Manual, ▇▇-▇▇▇▇▇-▇▇▇, for the detailed installation procedures. This form is to be used as a final verification to ensure that proper installation has been achieved after all procedures have been completed. This form must be completed by a NeuroStar-ce11ified Field Service Engineer. If any items in this form do not pass final inspection refer to the NeuroStar Distributor Service Manual, ▇▇-▇▇▇▇▇-▇▇▇, to correct the failure. The NeuroStar System is considered correctly installed when all items on this checklist are completed and all criteria are passed. |
| Mobile Console and Coil | Completed | Pass/Fail Criteria | Verification (Pass/Fail) |
Comments | Initial & Date | |||||
| Mobile Console System Self-Test | ☐ | All start-up tests display “pass” | ||||||||
| Coil Test Pulse passes left side | ☐ | Test-pulse test displays “Magnetic Field Strength Pass” | ||||||||
| LCD display is calibrated correctly | ☐ | LCD screen element accurately activates on touch |
||||||||
| Mobile Console Stop button (Emergency Stop Button) | ☐ | System stops when LCD stop button is touched | ||||||||
| Full range movement of Gantry | ☐ | Brakes energize to allow full range of movement of Gantry to mechanical stop | ||||||||
| Wheels lock properly on the Mobile Console | ☐ | Locking mechanism engages and wheels are unable to move | ||||||||
| Coil Output Test (WI 26-20024-000) | ☐ | Pulse Width is ³ 182µs Test Result µs |
||||||||
| Visual Inspection of Mobile Console and Coil conducted per Visual Inspection Procedure | ☐ | Completed procedure with no issues | ||||||||
| Treatment Chair | Completed | Pass/Fail Criteria | Verification (Pass/Fail) |
Comments | Initial & Date | |||||
| Memory Settings for M1 and M2 | ☐ | Chair executes command correctly and passes angle check | ||||||||
| Visual Inspection of Chair conducted per Visual Inspection Procedure | ☐ | Completed procedure with no issues | ||||||||
| Head Support | Completed | Pass/Fail Criteria | Verification (Pass/Fail) |
Comments | Initial & Date | |||||
| A/P Bar Laser | ☐ | Laser remains energized for a period of 30-45 seconds | ||||||||
| Full range of motion | ☐ | SOA, LC, A/P Bar, and front/back support achieve motion to mechanical stop | ||||||||
| Visual Inspection of Head Support conducted per Visual Inspection Procedure | ☐ | Completed procedure with no issues | ||||||||
| Computer / Software | Completed | Pass/Fail Criteria | Verification (Pass/Fail) |
Comments | Initial & Date | |||||
| Customer computer with TrakStar software installed | ☐ | Verify version is correct | ||||||||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
Form completion
| Completed By: | Title: | Date: |
Authorized Representative or DMAH Retains Form
| Completed By: | Title: | Date: |
Neuronetics Retains Form
| Completed By: | Title: | Date: |
Return form to Neuronetics Technical Support via email to ▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇.▇▇▇
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE L
Certificate of Conformance
See Attached
[*Three Pages Redacted*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE M
Specifications
See Attached
[*Twenty-Four Pages Redacted*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE N
Track Computer Specifications
| Processor | Pentium M or Pentium 4 at 1GHz or faster | |
| Operation System | Windows 7 Professional or later | |
| RAM | 2GB (min) | |
| Hard Drive | 60GB HD @5400 rpm (min) | |
| ROM Drive | 48x CD ROM (min) | |
| Graphics | 1024 x 768 minimum resolution
32 bit color | |
| Display/Monitor | 19” LCD Flat Panel (min) | |
| Web Browser | Google Chrome | |
| Internet | Internet access with separate network card and anti-virus software. | |
| Network | Isolated, secure, wired networking with NeuroStar(s) | |
| Accessories | Standard keyboard, mouse, printer | |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
SCHEDULE O
Accessories And Service Parts
| Replacement NeuroStar Product Accessories
| ||||||
| Catalog Number |
Item Number |
Description |
US$ transfer Price | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| Accessories
| ||||||
| Catalog Number |
Item Number |
Description |
US$ transfer Price | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| Service Parts
| ||||||
| Catalog Number |
Item Number |
Description |
US$ transfer Price | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] |
[*] | |||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] | |||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] | ||||
| [*] |
[*] |
[*] |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.

