SUPPLY AGREEMENT
| WESTAIM BIOMEDICAL CORP., a corporation incorporated under the laws of Alberta | |||
| (hereinafter referred to as “Westaim”) |
| XXXXX & NEPHEW INC., a corporation incorporated under the laws of Canada, XXXXX & NEPHEW, INC., a corporation incorporated under the laws of the State of Delaware in the United States of America, and X.X. XXXXX & NEPHEW LIMITED, a corporation formed and organized under the laws of England and Wales | |||
| (hereinafter collectively referred to as “S&N”) |
DEFINITIONS
| 1.1.1 | “Acticoat Absorbent Dressing Product” means the Acticoat absorbent dressing product (referred to in FDA 510 K number K002896) which has the features |
- 2 -
| set out in the Specifications set out in Schedule A and which was marketed and sold by Westaim under the tradename “Acticoat Absorbent Dressing” immediately prior to the Effective Date, together with all Improvements thereto which Westaim and S&N agree in writing to implement; | |||
| 1.1.2 | “Acticoat Moisture Control Dressing Product” means the Acticoat moisture control dressing product (referred to in FDA 510 K number K010447) which has the features set out in the Specifications set out in Schedule B and which was marketed and sold by Westaim under the tradename “Acticoat Moisture Control Dressing” immediately prior to the Effective Date, together with all Improvements thereto which Westaim and S&N agree in writing to implement; | ||
| 1.1.3 | “Acticoat Product” means the Acticoat product (referred to in FDA 510 K numbers K955453) which has the features set out in the Specifications set out in Schedule C and which was marketed and sold by Westaim under the tradename “Acticoat” or “Acticoat Burn Dressing” immediately prior to the Effective Date, together with all Improvements thereto which Westaim and S&N agree in writing to implement; | ||
| 1.1.4 | “Acticoat 7 Product” means the Acticoat product (referred to in FDA 510 K number K001519) which has the features set out in the Specifications set out in Schedule D and which was marketed and sold by Westaim under the tradename “Acticoat 7” immediately prior to the Effective Date, together with all Improvements thereto which Westaim and S&N agree in writing to implement; | ||
| 1.1.5 | “Affiliates” means any Person that directly or indirectly controls, is controlled by, or is under common control with another Person. A Person shall be deemed to “control” another business entity if it owns, directly or indirectly, more than fifty percent (50%) of the outstanding voting securities, capital stock, partnership interest or other comparable equity or ownership interest of such Person. If the laws of the jurisdiction in which such Person operates prohibit ownership of more than fifty percent (50%), control shall be deemed to exist at the maximum level of ownership allowed by such jurisdiction; | ||
| 1.1.6 | “Agreement” means this supply agreement, together with all schedules hereto and any amendments to or restatements of this supply agreement; | ||
| 1.1.7 | “Asset Purchase Agreement” means the asset purchase agreement of even date herewith between Westaim and Xxxxx & Nephew, Inc., together with all Schedules thereto and any amendments to or restatements of such asset purchase agreement; | ||
| 1.1.8 | “Business Day” means any day except a day that is a Saturday, a Sunday or a statutory holiday in Alberta; | ||
| 1.1.9 | “Claim” has the meaning attributed to that term in Section 9.4; |
- 3 -
| 1.1.10 | “Confidential Information” has the meaning attributed to that term in Section 8.1; | ||
| 1.1.11 | “Distribution Royalties” has the meaning attributed to that term in the License and Development Agreement; | ||
| 1.1.12 | “Effective Date” means 11:59 pm Calgary time on the 8th day of May, 2001, or such other time and date as the parties to this Agreement may agree upon in writing; | ||
| 1.1.13 | “Equipment” means the equipment listed in Schedule E and any New Equipment which subsequently becomes Equipment as provided in Section 11.11; | ||
| 1.1.14 | “Escrow Agreement” means the manufacturing, technology, escrow agreement of even date herewith among S&N, Westaim, Westaim Biomedical Inc., and Montreal Trust Company of Canada, together with all schedules thereto and any amendments to or restatements of such manufacturing technology escrow agreement; | ||
| 1.1.15 | “Event Milestone Payments” means payments under Section 6.4 of the License and Development Agreement; | ||
| 1.1.16 | “FDA” means the United States Food and Drug Administration, or any successor organization; | ||
| 1.1.17 | “Field” has the meaning attributed to that term in the License and Development Agreement; | ||
| 1.1.18 | “First Refusal Free Transfer Period” has the meaning attributed to that term in Section 12.7; | ||
| 1.1.19 | “First Refusal Transfer Closing Date” has the meaning attributed to that term in Section 12.7; | ||
| 1.1.20 | “First Refusal Transfer Notice” has the meaning attributed to that term in Section 12.7; | ||
| 1.1.21 | “First Refusal Transfer Period” has the meaning attributed to that term in Section 12.7; | ||
| 1.1.22 | “Fixed Cost Portion of Fully Allocated Cost of Goods” means the portion of Fully Allocated Cost of Goods (without regard for the proviso in such definition concerning the limitation on the increase of the Fixed Cost Portion of Fully Allocated Cost of Goods) that does not relate in any way to: variable costs, including, without limitation, costs relating to raw materials and any other costs which increase or decrease depending upon the volume of Product produced; costs not within the control of Westaim or its Affiliates, including |
- 4 -
| without limitation the costs of supplies or services provided by third parties; costs incurred as a result of complying with an order or request of a governmental authority or regulatory body that relates to manufacturing or the manufacturing facilities; costs incurred at the request of S&N; and any costs resulting from increases in the amount of compensation to employees of Westaim or its Affiliates if such increase was a part of a general increase in the amount of compensation to employees in similar positions within Westaim; |
| 1.1.23 | “Force Majeure Event” means the events described in Section 12.1; | ||
| 1.1.24 | “Free Transfer Period” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.25 | “Fully Allocated Cost of Goods” means with respect to the manufacture and supply of a particular Product, the aggregate of |
| 1.1.25.1 | direct material, direct labour and subcontracted costs incurred by Westaim in connection with the procurement of raw materials or production, manufacture, processing, labeling, testing, transportation and packaging of such Product, and | ||
| 1.1.25.2 | S&N’s proportionate share of indirect costs incurred by Westaim relating to the manufacturing and manufacturing facilities (“Manufacturing Costs”) related to such Product including administration and labour (in either case relating to the manufacturing), depreciation on equipment owned by Westaim, rent, insurance, utilities, taxes (excluding taxes on income), repairs, maintenance, cleaning, training, quality control and the like, with such calculation to be made in accordance with Canadian GAAP. S&N’s proportionate share of such indirect costs shall be based on the percentage of the aggregate Manufacturing Costs in the reporting period for which S&N is responsible under this Agreement, being, for Equipment (other than New Equipment) [***] (or [***] if Westaim has relinquished its [***] reservation of capacity) less the proportion of Production Capacity (in excess of any such reserve capacity, if applicable) actually used by Westaim in the reporting period for purposes authorized by this Agreement, and for New Equipment, the actual usage of such New Equipment by Westaim in the reporting period for purposes authorized by this Agreement as a percentage of actual use by Westaim for other purposes. |
| provided, however, that for purposes of this Agreement, the Fixed Cost Portion of Fully Allocated Cost of Goods shall not rise from one calendar year to the next by a percentage amount that exceeds the greater of [***] and the percentage increase in the Consumer Price Index, as published by Statistics Canada, from such calendar year to the next; | |||
| 1.1.26 | “Governmental Permits” has the meaning attributed to that term in Section 2.4; |
- 5 -
| 1.1.27 | “Improvements” has the meaning attributed to that term in the License and Development Agreement; | ||
| 1.1.28 | “Knowledge” means, in the case of S&N the actual knowledge of Xxxxxxx Xxxx, Xxxxx Xxxxxxx, Xxx Xxxxx, Xxxxxx Xxxxxx and Xxxxxx Xxxxx after reasonable inquiry, and, in the case of Westaim, means the actual knowledge of Xxxxx Xxxx, Xxxxx Xxxxxx and Xxxxxxx Vatelero, after reasonable inquiry; | ||
| 1.1.29 | “License and Development Agreement” means the license and development agreement of even date herewith among Westaim, Westaim Biomedical Inc., X.X. Xxxxx & Nephew Limited and Xxxxx & Nephew, Inc., together with all schedules thereto and any amendments to or restatements of such agreement; | ||
| 1.1.30 | “Lien” means, with respect to any property, any assignment, mortgage, charge, pledge, lien, hypothec, conditional sale or title retention agreement, lease, levy, execution, seizure, attachment, garnishment or other similar encumbrance or security interest in respect of such property, howsoever arising (including, without limitation, pursuant to applicable law), whether absolute or contingent, fixed or floating, legal or equitable, perfected or otherwise; | ||
| 1.1.31 | “Manufacturing Assets” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.32 | “Manufacturing License” has the meaning attributed to that term in Section 4.1.1.1; | ||
| 1.1.33 | “Negotiation Period” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.34 | “Net Sales” has the meaning attributed to that term in the License and Development Agreement; | ||
| 1.1.35 | “New Equipment” has the meaning attributed to that term in Section 11.11; | ||
| 1.1.36 | “New Product” means any new product that the parties agree in writing to add to the scope of this Agreement, with mutually agreed upon Specifications to be evidenced in writing by the appending of an additional Schedule to this Agreement; | ||
| 1.1.37 | “Offered Purchase Price” has the meaning attributed to that term in Section 12.7; | ||
| 1.1.38 | “Offered Purchase Terms” has the meaning attributed to that term in Section 12.7; | ||
| 1.1.39 | “Party” means Westaim or S&N, as the case may be, and “Parties” means Westaim and S&N; | ||
| 1.1.40 | “Percentage Manufacturing Profit” means [***] of |
- 6 -
| Net Sales for all Products until the earlier of: (i) May 8, 2004, and (ii) the first day of the calendar quarter immediately following four consecutive calendar quarters during which Net Sales equaled at least [***], in the aggregate, whereupon “Percentage Manufacturing Profit” means [***] of Net Sales for all Products; |
| 1.1.41 | “Person” means an individual, corporation, company, cooperative, partnership, trust, unincorporated association, entity with juridical personality or a governmental authority or body, and pronouns which refer to a Person have a similarly extended meaning; | ||
| 1.1.42 | “Production Capacity” means the manufacturing capacity of the Equipment, reduced to take into account all down times including scheduled and unscheduled maintenance; | ||
| 1.1.43 | “Products” means the Acticoat Product, the Acticoat 7 Product, the Acticoat Absorbent Dressing Product, the Acticoat Moisture Control Dressing Product, and includes any inventory of such Products existing as at the Effective Date, and any New Product and “Product” means any one of the Products; | ||
| 1.1.44 | “Purchase Option” has the meaning attributed to that term in Section 12.7; | ||
| 1.1.45 | “Qualifying Offer” has the meaning attributed to that term in Section 12.7; | ||
| 1.1.46 | “Regulatory Authority” means, with respect to a particular Product, those government agencies or authorities responsible for the approval of such Product in the United States, Canada and the European Union (taken as a whole, not individual member countries); | ||
| 1.1.47 | “Rejection Date” has the meaning attributed to that term in Section 2.2.3; | ||
| 1.1.48 | “Rejection Notice” has the meaning attributed to that term in Section 2.2.3; | ||
| 1.1.49 | “S&N Indemnities” has the meaning attributed to that term in Section 9.1; | ||
| 1.1.50 | “S&N Offer” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.51 | “S&N Production Capacity” has the meaning attributed to that term in Section 11.3; | ||
| 1.1.52 | “S&N Purchase Price” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.53 | “S&N Purchase Terms” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.54 | “Sales Milestone Payments” means payments made to Westaim under Section 6.5 of the License and Development Agreement; | ||
| 1.1.55 | “Sales Report” has the meaning attributed to that term in Section 3.2; |
- 7 -
| 1.1.56 | “Specifications” means, with respect to a particular Product, the specifications in relation to such Product set forth in the applicable Schedule for that Product to this Agreement or, with respect to a New Product, as agreed upon in writing by Westaim and S&N, as such specifications may be amended from time to time by the written agreement of Westaim and S&N; | ||
| 1.1.57 | “Survival Period” has the meaning attributed to that term in Section 12.9; | ||
| 1.1.58 | “Term” means the term of this Agreement as specified in Section 10.1; | ||
| 1.1.59 | “Territory” means, subject to Sections 1.1.16.13 and 1.1.16.14 of the License and Development Agreement, all countries of the world; | ||
| 1.1.60 | “Transfer Closing Date” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.61 | “Transfer Notice” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.62 | “Transfer Period” has the meaning attributed to that term in Section 12.6; | ||
| 1.1.63 | “Transition Services Agreement” means the transition services agreement of even date herewith among Westaim and Xxxxx & Nephew Inc. and Xxxxx & Nephew, Inc., together with all schedules thereto and any amendments to or restatements of such transition services agreement; | ||
| 1.1.64 | “Westaim Manufacturing Technology” means, with respect to a particular Product in the Field, the proprietary technology relating to the manufacture of such Product owned by Westaim or licensed in by Westaim with the right to sublicense in the manner contemplated by this Agreement and which is necessary in connection with the manufacture of such Product; | ||
| 1.1.65 | “Westaim Know-How” has the meaning attributed to that term in the License and Development Agreement; | ||
| 1.1.66 | “Westaim Indemnities” has the meaning attributed to that term in Section 9.2; | ||
| 1.1.67 | “Westaim Patent Rights” has the meaning attributed to that term in the License and Development Agreement; and | ||
| 1.1.68 | “Westaim Production Capacity” has the meaning attributed to that term in Section 11.3. |
- 8 -
- 9 -
MANUFACTURING AND SUPPLY OBLIGATIONS
| 2.2.1 | Westaim agrees to supply to S&N and S&N agrees to purchase exclusively from Westaim such quantities of such Product as S&N shall request monthly by written purchase order, all in accordance with the terms of this Agreement. S&N shall, on a monthly basis during the Term (except during any period in which S&N is exercising its rights under the Manufacturing License in accordance with Article 4), provide Westaim with a twelve (12) month non-binding rolling forecast of S&N’s estimated requirements for the Product. Such twelve (12) month non-binding rolling forecast shall be provided to Westaim no later than five (5) Business Days preceding the beginning of each calendar month and shall cover the twelve (12) month period commencing with the calendar month next following such calendar month (e.g., on or before September 21, 2001, S&N is required to provide Westaim with an updated twelve (12) month rolling non-binding forecast for the period November 1, 2001 to October 31, 2002). S&N shall on a monthly basis request a supply of the Product from Westaim by way of written purchase order. The purchase order for a particular month shall be delivered to Westaim no later than five (5) Business Days preceding the beginning of the particular month. The minimum amount of the Product ordered by S&N for any particular month shall be [***] of the requirements last estimated for such month in the applicable twelve (12) month non-binding rolling forecast. Each purchase order shall be binding on S& N and Westaim, and Westaim shall fill each purchase order of S&N within [***] days from receipt of such purchase order, provided, however, that, with respect to a particular month, in the event S&N’s purchase order exceeds [***] of the requirements last estimated for such month in the applicable twelve (12) month non-binding rolling forecast, Westaim shall not be obligated to fill the amount of such purchase order in excess of [***] but shall only be required to use reasonable commercial efforts to fill the amount of such purchase order in excess of the requirements last |
- 10 -
| estimated for such month in the applicable twelve (12) month non-binding rolling forecast. Further, notwithstanding the above, but subject to Westaim complying with Section 11.11, under no circumstances shall Westaim be obligated to supply a quantity of Products over any period that exceeds the quantity of Products that can be produced by the Equipment during such period using the S&N Production Capacity; | |||
| 2.2.2 | all Products purchased hereunder by S&N from Westaim shall conform to the applicable Specifications at the time of delivery by Westaim to S&N; and | ||
| 2.2.3 | upon receipt of any Product, S&N may inspect and test such Products for conformance to the applicable Specifications and may, up to and including the date which is thirty (30) days after the date of receipt by S&N of such Products (the “Rejection Date”), reject the same by sending to Westaim written notice (the “Rejection Notice”) of such rejection on or before the Rejection Date (specifying the nature of such non-conformance) if such Product does not conform to the Specifications. S&N shall be deemed to have accepted a particular delivery of such Product if S&N has not sent to Westaim a Rejection Notice in connection with such delivery on or before the Rejection Date related to such delivery; provided, however, that S&N shall have the right to reject Product by delivery to Westaim of a Rejection Notice after the Rejection Date, as soon as is practicable following S&N becoming aware of the non- conformance of the Product, if such Product did not conform to the Specifications at the time it was delivered to S&N and the non-conformance resulted from a latent defect not capable of being detected by S&N during a reasonable inspection and testing of the Product at the time of delivery to S&N. If Westaim disagrees with the alleged non-conformity of such Product with the Specifications, and after the Parties have endeavoured to settle such disagreement between themselves, then an independent laboratory, mutually agreed upon in writing by the Parties (acting reasonably), shall analyze samples of the alleged non-conforming Product to determine compliance with the Specifications. Westaim and S&N shall be bound by the laboratory analysis of such alleged non-conformity of such Product with the Specifications. The cost incurred in connection with retaining the independent laboratory shall be borne by S&N if the Product in question is found to conform to the Specifications and by Westaim if the Product in question is found to not conform to the Specifications. To the extent it is determined that any Product supplied by Westaim to S&N under this Agreement does not conform to the Specifications, Westaim’s sole obligation and S&N’s sole remedy shall be to have Westaim replace such non-conforming Product at no additional cost to S&N. | ||
| 2.2.4 | S&N and Westaim agree to negotiate in good faith to conclude a “technical agreement” required by the Regulatory Authorities in respect of a Product within ninety (90) days of the Effective Date, and to negotiate in good faith to conclude a “technical agreement” required by the Regulatory Authorities in respect of a New Product prior to the anticipated launch of such New Product. |
- 11 -
| 2.4.1 | Westaim represents and warrants to S&N that each Product will be produced in compliance with all applicable laws, the non-compliance with which would have a material adverse effect on Westaim’s ability to meet its obligations under this Agreement in relation to such Product. Westaim shall at all times relevant throughout the Term own, hold or possess all material licenses, franchises, permits, privileges, immunities, registrations, approvals (including, without limitation, regulatory approvals), authorizations and qualifications (“Government Permits”) necessary to allow it to manufacture and produce the Products. All such Government Permits shall in all material respects be valid, subsisting and in good standing at all relevant times throughout the Term. | ||
| 2.4.2 | Westaim represents and warrants that it is the owner of the Westaim Patent Rights and that such ownership is unencumbered by any lien or security interest granted by Westaim and, to the Knowledge of Westaim, it is the owner of the Westaim Know-How and that all material formal requirements in connection with the maintenance and continuation of the patents and trade-marks (including the payment of fees and taxes necessary for the continued use of and/or registration thereof) have been complied with as at the Effective Date. Westaim has no Knowledge of any reason why any patent included in the Westaim Patent Rights would be held to be invalid or otherwise unenforceable, of any reason why any patent application included in the Westaim Patent Rights would be refused or withdrawn, of any reason why any Person would claim that the production, use or sale of Products would infringe its intellectual property rights or that any trade secret of another Person has been appropriated by Westaim in the production of the Products. Westaim has taken reasonable measures to avoid misappropriation of trade secrets or infringement of copyright. For purposes of this Section 2.4.2, it is acknowledged that “ownership” goes to title and not to right to use or exploit, and that title does not necessarily give the right to use or otherwise exploit. | ||
| 2.4.3 | Except as expressly provided in this Article 2, Westaim makes no representation or warranty, whether express or implied, with respect to the Products including, without limitation, any representation as to fitness for a particular purpose or merchantable quality. |
- 12 -
- 13 -
CONSIDERATION
- 14 -
| 3.3.1 | Westaim will keep and maintain proper and complete records and books of account in such form and detail as is necessary for the determination of the Fully Allocated Cost of Goods. Westaim shall once in each calendar year during normal business hours and upon fifteen (15) days prior notice from S&N make those records available for audit by a nationally recognized accounting firm designated by S&N (except one to which Westaim shall have objection, acting reasonably) for the sole purpose of, and Westaim will only be required to disclose information related to, verifying the Fully Allocated Cost of Goods and the correctness of calculations and classifications in respect thereof. Westaim shall preserve such records made in any calendar year for a period of seven (7) years following the close of that calendar year. In the event that such audit discloses that the actual amount of Fully Allocated Cost of Goods are less than the amount paid by S&N to Westaim pursuant to this Article 3, then Westaim shall promptly reimburse to S&N such overpayment. In the event that such audit discloses that the actual amount of Fully Allocated Cost of Goods payable by S&N to Westaim are greater than the amount paid by S&N to Westaim pursuant to this Article 3, then S&N shall promptly pay to Westaim such underpayment based on the results disclosed by such audit. The cost of such audit shall be borne by S&N unless such audit discloses that Fully Allocated Cost of Goods is less by [***] or more than the amount paid by S&N to Westaim pursuant to this Article 3 or such audit discloses that Fully Allocated Cost of Goods is more than the amount paid by S&N to Westaim pursuant to this Article 3, in which case Westaim shall be responsible for payment of all reasonable costs of such audit to a maximum of the amount of any underpayment by S&N to Westaim due to an incorrect calculation of Fully Allocated Cost of Goods and S&N shall be responsible for payment of all other costs of such audit. Notwithstanding the foregoing, S&N shall not have the right to conduct more than once, for the same purpose, an audit of the same information, books and records, whether under this Agreement or the License and Development Agreement; provided, however, that if any such audit discloses that the actual Fully Allocated Cost of Goods was calculated incorrectly such that a reimbursement by Westaim is required pursuant to this Section 3.3.1, then S&N shall have a further right to audit the same information, books and records for the same purpose until such time as no further errors are found. | ||
| 3.3.2 | S&N will keep and maintain proper and complete records and books of account in such form and detail as is necessary for the determination of the Net Sales amounts payable by S&N to Westaim under this Agreement. S&N shall once in each calendar year during normal business hours upon fifteen (15) days prior notice from Westaim make those records available for audit by a nationally recognized accounting firm designated by Westaim (except one to which S&N shall have objection, acting reasonably) for the sole purpose of, and S&N will only be required to disclose information related to, verifying such Net Sales and deductions therefrom and the correctness of calculations |
- 15 -
| and classifications in respect thereof. S&N shall preserve such records made in any calendar year for a period of seven (7) years following the close of that calendar year. In the event that such audit discloses that the actual Net Sales amounts for Products are greater than the Net Sales amounts reported by S&N to Westaim pursuant to this Article 3 for purposes of calculating the purchase price payable by S&N to Westaim for Products, then S&N shall pay to Westaim any additional purchase price for Products based on the results disclosed by such audit. In the event that such audit discloses that the actual Net Sales amounts for Products are less than the Net Sales amounts reported by S&N to Westaim pursuant to this Article 3, then Westaim shall reimburse S&N for any such overpayment of the purchase price for Products based on the results disclosed by such audit. The cost of such audit shall be borne by Westaim unless such audit discloses that the actual Net Sales amounts for Products are greater by [***] or more than the Net Sales amounts reported by S&N to Westaim pursuant to this Article 3 or such audit discloses that the Net Sales for Products are less than the Net Sales reported by S&N to Westaim pursuant to this Article 3, in which cases S&N shall be responsible for payment of all reasonable costs of such audit to a maximum of the amount of any overpayment by S&N to Westaim due to an incorrect calculation of Net Sales and Westaim shall be responsible for payment of all other costs of such audit. Notwithstanding the foregoing, Westaim shall not have the right to conduct more than once, for the same purpose, an audit of the same information, books and records, whether under this Agreement or the License and Development Agreement; provided, however, that if any such audit discloses that the actual Net Sales was calculated incorrectly such that a payment by S&N is required pursuant to this Section 3.3.2, then Westaim shall have a further right to audit the same information, books and records for the same purpose until such time as no further errors are found. |
- 16 -
| 3.6.1 | deduct those taxes or other amounts from the amount of such payment due to the receiving Party, | ||
| 3.6.2 | pay the taxes or other amounts to the proper taxing authority in a timely manner, and | ||
| 3.6.3 | send proof of payment to the receiving Party within sixty (60) days following that payment. |
MANUFACTURING LICENSE
| 4.1.1 | License to Make and Have Made in the Territory |
| 4.1.1.1 | With respect to Products, Westaim hereby grants to X.X. Xxxxx & Nephew Limited and its Affiliates a royalty-free license (or, where applicable, sublicense) under the Westaim Manufacturing Technology to make and have made Products for sale by S&N in the Field in the Territory pursuant to the License and Development Agreement (the “Manufacturing License”); provided, however, that S&N may not practice the Manufacturing License until such time, if ever, as Westaim |
- 17 -
| has failed to cure a material supply difficulty or failed to satisfy S&N, acting reasonably, that it can cure such supply difficulty at least as well as S&N, all as described in Section 4.1.1.3. | |||
| 4.1.1.2 | Notwithstanding the foregoing, Westaim agrees to advise S&N in a timely manner of any material supply difficulties (which shall include the inability or unwillingness of Westaim to provide assistance to S&N pursuant to Section 11.11) with respect to a Product or Products experienced by Westaim (including any caused by a Force Majeure Event) so that the Parties have an opportunity to discuss such difficulties and possible resolutions thereof at an early stage with a view to avoiding the application of the Manufacturing License in relation to the Product or Products. | ||
| 4.1.1.3 | If Westaim advises S&N of a supply difficulty pursuant to Section 4.1.1.2 or encounters one but does not notify S&N thereof and S&N desires to practice the Manufacturing License with respect to Products (in whole or in part), S&N will notify Westaim in writing of such desire not earlier than [***] following the commencement of the discussions referred to in Section 4.1.1.2 or, if no discussions occur because Westaim failed to notify S&N of the supply difficulty, [***] following the date S&N becomes aware of the supply difficulty. In that event, Westaim shall have a period of [***] following receipt of the notice from S&N to either cure the supply difficulty or demonstrate to the satisfaction of S&N, acting reasonably, that it can cure the supply difficulty at least as well as S&N, having regard for the time and cost to implement the cure. If, by the end of the [***] period, and only in such circumstances, Westaim has neither cured the supply difficulty nor demonstrated to the satisfaction of S&N, acting reasonably, that it can cure such difficulty at least as well as S&N, then S&N shall become entitled to practice the Manufacturing License with respect to such Product to the extent requested by S&N in its written notice to Westaim. Except as expressly provided in Section 9.1 (insofar as it pertains to third party claims against S&N), the right to practice the Manufacturing License in relation to such Product will be S&N’s sole remedy for the actions of Westaim giving rise to that right. S&N shall purchase from Westaim such quantity of such Product and material intermediates as are then held by or on behalf of Westaim. | ||
| 4.1.1.4 | During any period in which S&N is practicing the Manufacturing License with respect to Products, S&N shall: |
| 4.1.1.4.1 | be responsible for all aspects of the manufacture and supply of such Products assumed by S&N and, as a result, Westaim shall, notwithstanding any provision to the contrary in this Agreement, be relieved from all obligations |
- 18 -
| under this Agreement with respect to the manufacture and supply of such Products during such period, including without limitation any liability in respect thereof to S&N or any third party, except in relation to Products manufactured and supplied by Westaim prior to the commencement of such period or after the conclusion of such period; | |||
| 4.1.1.4.2 | only modify the label of such Product to the extent required by the applicable Regulatory Authority. |
| 4.1.1.5 | At the time that S&N is entitled to practice the Manufacturing License under this Section 4.1, the lease provided for in Article 11 shall be deemed to have been terminated, and Westaim shall comply with the provisions in Section 11.8 with respect to expiry of the Term and the return of the Equipment. | ||
| 4.1.1.6 | If within one year after S&N begins to practice the Manufacturing License with respect to such Products in the Field in the Territory (to the extent requested by S&N in its written notice to Westaim), Westaim has demonstrated to the satisfaction of S&N, acting reasonably, the ability to once again manufacture such Products for sale in the Territory in compliance with the provisions of this Agreement at a Fully Allocated Cost of Goods no more than the Fully Allocated Cost of Goods incurred by S&N during its period of manufacture, and has paid to S&N an amount equal to all of the costs directly incurred by S&N in establishing and terminating such manufacturing operations (including all capital expenditures or commitments to third parties in relation thereto made by S&N to enable it to manufacture and supply such Products), S&N shall, subject to then existing third party commitments, permit Westaim to resume such manufacturing activities of such Products for sale in the Territory. S&N will use commercially reasonable efforts in structuring any third party arrangements to minimize the duration and scope of any such commitments so as to facilitate the resumption by Westaim of manufacturing activities of such Products for sale in the Field in the Territory. Upon such resumption by Westaim, the lease provided for in Article 11 shall resume and S&N will no longer be entitled to practice the Manufacturing License with respect to such Products in the Territory (except to the limited extent required to enable S&N to comply with then existing third party commitments regarding manufacture of such Products) until such tune as the conditions described in Section 4.1.1.2 above recur and the procedures in Section 4.1.1.3 above have once again been complied with and S&N is thereby entitled to again practice the Manufacturing License. |
- 19 -
| 4.1.2 | Technology Transfer |
| 4.1.2.1 | In order to enable S&N to effectively exercise its rights under the Manufacturing License when applicable, Westaim, S&N and certain other named parties have concurrently with this Agreement entered into the Escrow Agreement. Further, in the event that S&N becomes entitled to practice the Manufacturing License with respect to Products, Westaim will provide S&N with all assistance reasonably required to ensure a smooth and seamless transition of manufacturing of Products, including the ability to consult with Westaim personnel involved in manufacturing Products and, if Westaim has provided its prior written consent, the ability to make offers of employment to such personnel. | ||
| 4.1.2.2 | Any information received by S&N pursuant to Section 4.1.2 shall be subject to the confidentiality provisions of Article 8 and shall be deemed to be the Confidential Information of both S&N and Westaim. S&N shall return any such information to Westaim at such time as its ability to practice the Manufacturing License with respect to Products terminates (except that S&N may retain one copy for legal archival purposes). |
| 4.1.3 | Protection in Bankruptcy. The rights granted to S&N by Westaim pursuant to this Section 4.1 constitute “intellectual property” (including as such term is defined under Section 101 (35A) of the United States Bankruptcy Code) for purposes of applicable bankruptcy law (including Section 365(n) of the United States Bankruptcy Code). | ||
| 4.1.4 | Force Majeure Events |
- 20 -
RECALLS AND COMPLAINTS
INSURANCE
| 6.1.1 | Subject to adjustment as hereinafter provided, Westaim shall, at its sole cost and expense, procure and maintain policies of commercial general liability insurance in amounts of not less than [***] per incident and [***] annual aggregate and naming S&N as an additional insured and of business interruption insurance in amounts of not less than two million dollars ($2,000,000) per incident and ten million dollars ($10,000,000) annual aggregate and naming S&N as an |
- 21 -
| additional insured. Such commercial general liability insurance shall, among other things, provide (a) product liability coverage and (b) contractual liability coverage for Westaim’s indemnification under Section 9.1. If Westaim elects to self-insure all or part of the limits described above (including deductibles or retentions which are in excess of [***] annual aggregate), such self-insurance program must be reasonably acceptable to S&N. The minimum amounts of insurance coverage required under these provisions shall not be construed to create a limit of Westaim’s liability with respect to its indemnification obligation under Section 9.1. S&N and Westaim agree to review on the fifth, tenth, fifteenth, etc., anniversary of the Effective Date the adequacy of the insurance requirements set forth in this Section 6.1.1 in the then current circumstances and to revise such requirements where appropriate, acting reasonably, to conform with the then current industry norms. | |||
| 6.1.2 | Subject to adjustment as hereinafter provided, S&N shall, at its sole cost and expense, procure and maintain policies of commercial general liability insurance in amounts of not less than [***] per incident and [***] annual aggregate and naming Westaim as an additional insured and of business interruption insurance in amounts of not less than two million dollars ($2,000,000) per incident and ten million dollars ($10,000,000) annual aggregate and naming Westaim as an additional insured. Such commercial general liability insurance shall, among other things, provide (a) product liability coverage and (b) contractual liability coverage for S&N’s indemnification under Section 9.2. If S&N elects to self-insure all or part of the limits described above (including deductibles or retentions which are in excess of [***] annual aggregate), such self-insurance program must be reasonably acceptable to Westaim. The minimum amounts of insurance coverage required under these provisions shall not be construed to create a limit of S&N’s liability with respect to its indemnification obligation under Section 9.2. S&N and Westaim agree to review on the fifth, tenth, fifteenth, etc., anniversary of the Effective Date the adequacy of the insurance requirements set forth in this Section 6.1.2 in the then current circumstances and to revise such requirements where appropriate, acting reasonably, to conform with the then current industry norms. |
- 22 -
IMPROVEMENTS
CONFIDENTIALITY
| 8.1.1 | Except as otherwise expressly authorized in writing by the discloser or specifically provided for in this Agreement, all Confidential Information of the disclosing Party shall be held in strict confidence by the receiving Party and the receiving Party shall employ or cause to be employed diligent efforts and reasonable care in order to ensure that such Confidential Information is not made available to any third Party, excepting only as required under this Agreement including in connection with the exercise by S&N of its rights in relation to the Manufacturing License, where applicable or to the directors, officers, employees, agents and consultants of the receiving Party whose duties require disclosure of the same or as expressly authorized in writing and then (in any such case) only if the Parties to whom such Confidential Information is being disclosed have given to the disclosing Party an enforceable undertaking (in a form of contract used by the disclosing Party in its normal course of business and that that deals with equivalently sensitive information) not to disclose such Confidential Information to any other Party. | ||
| 8.1.2 | Subject to Section 8.1.3, Confidential Information shall not include information that is in the public domain at the time of disclosure, that the recipient can demonstrate based on written records was lawfully already in its possession, that is approved in writing for release by the discloser or that is obtained by any Party to this Agreement from a third Party without obligation |
- 23 -
| of confidence (provided, however, that no third Party from which the information is obtained has any obligation of confidence to any Parties to this Agreement) or that becomes public knowledge otherwise than through the fault of the recipient or any Person to whom it has disclosed the Confidential Information. | |||
| 8.1.3 | Disclosure of Confidential Information shall not be precluded if such disclosure is in response to a valid order of a court or other governmental body or of any political subdivision thereof or is otherwise required to be disclosed by law. Notwithstanding the foregoing, in the event that a Party is required to make a disclosure of the other Parry’s Confidential Information pursuant to this Section 8.1.3, it will, except where impractical, give reasonable advance notice to the other Party of such disclosure and use its best efforts to secure confidential treatment of such information. | ||
| 8.1.4 | Confidential Information that is specific shall not be deemed to be within the public domain merely because it is embraced by general knowledge in the public domain. Further, any Confidential Information dealing with any combination of features of a specific matter shall not be deemed to be within the public domain, unless the combination of features and the principle of operation are in the public domain. | ||
| 8.1.5 | Each Party to this Agreement shall, upon the termination of this Agreement, return all corporeal Confidential Information to the owner of such Confidential Information with a written undertaking that no copies (electronic or otherwise) have been retained, except that the receiving Party may retain one (1) copy in its legal files solely to allow it to monitor its obligations hereunder. | ||
| 8.1.6 | Each of the Parties to this Agreement acknowledges that the other Party to this Agreement would suffer irreparable harm as a result of the breach of any of the non-disclosure and confidentiality obligations set forth in this Section 8.1 and that legal remedies are inadequate; therefore, each of the Parties to this Agreement agrees that, in addition to any damages and other remedies that the other Party to this Agreement may be entitled to as a result of such a breach, the other Party to this Agreement shall be entitled to seek an order from a court of competent jurisdiction restraining such Party from breaching or continuing to breach any of the provisions of this Section 8.1. | ||
| 8.1.7 | The covenants of the Parties under this Section 8.1 shall continue in full force and effect notwithstanding the termination of this Agreement by effluxion of time or otherwise. |
- 24 -
INDEMNIFICATION
- 25 -
| 9.3.1 | procure the right for Westaim to continue using the allegedly infringing Westaim Manufacturing Technology; or | ||
| 9.3.2 | replace or modify the Westaim Manufacturing Technology, as the case may be, so that it becomes non-infringing but has substantially equivalent capabilities as the infringing technology. |
- 26 -
TERM AND TERMINATION
- 27 -
| (30) Business Days all or substantially all of its business operations, other than suspensions of a temporary nature resulting from a strike or similar event not within the control of the Party or from a lock-out. |
LEASE OF EQUIPMENT
- 28 -
- 29 -
| 11.6.1 | keep the Equipment free of all Liens of any kind or nature arising through Westaim; | ||
| 11.6.2 | not transfer, deliver up possession of, encumber or sublet the Equipment; | ||
| 11.6.3 | keep all Equipment in good repair, condition and working order and shall furnish all parts, mechanisms, devices and servicing so required, subject to Section 11.8 below; | ||
| 11.6.4 | comply with all laws, regulations and orders relating to the Equipment; and | ||
| 11.6.5 | pay all taxes associated with Westaim’s use of the Equipment. |
| 11.7.1 | For so long as the Equipment is in Westaim’s possession, Westaim assumes and shall bear the entire risk of loss, damage to or destruction of the Equipment from any cause whatsoever. Westaim shall obtain and maintain for the Term, at its own expense, insurance against loss or damage to the Equipment including without limitation, loss by fire (including extended coverage), theft, collision and such other risks of loss as are customarily covered by insurance on such type of Equipment and by prudent operators of businesses similar to that in which Westaim is engaged, in such amounts, in such form and with such insurers as shall be satisfactory to S&N, acting reasonably provided that the amount of such insurance shall not be less than the full replacement value of the Equipment. The insurance coverage shall be in favour of both S&N and Westaim as named insureds, shall name S&N as first loss payee, and shall contain a clause requiring the insurer to give S&N at least fifteen (15) business days prior written notice of any alteration in the terms of or cancellation of the policy. On request, Westaim shall furnish to S&N a certificate of insurance or other evidence satisfactory to S&N that such insurance coverage is in effect. Westaim shall give S&N prompt notice of any damage to or loss of the Equipment or any part thereof. | ||
| 11.7.2 | If there is any damage to or loss of any part of the Equipment, Westaim will at its expense make all proofs of loss and take all other steps necessary to recover insurance benefits, unless advised in writing by S&N that S&N desires to do so. Westaim appoints S&N as its agent and attorney to make any claims and receive any payments pursuant to the insurance policies. Proceeds of insurance will be disbursed by S&N against invoices for repair, partial repair or replacement of the Equipment. However, if any part of the Equipment is lost, stolen, destroyed or damaged beyond repair for any reason, the Parties shall determine in good faith discussions whether Westaim or S&N ought to replace the Equipment, such determination to be made in a manner substantially similar to that set out in Article 4 concerning curation of a material supply difficulty. |
- 30 -
| 11.10.1 | S&N does not make, and there are no representations or warranties, express or implied, statutory or otherwise, with respect to the Equipment or this Agreement or any schedule or amendment hereto or affecting the rights of the Parties hereby and S&N shall not be deemed to make, now or hereafter at any time, any representation or warranty, express or implied, as to the quality of the material or workmanship of the Equipment, or the conformity of the Equipment to the provisions and specifications of Westaim, or to the condition, design, merchantable quality, durability, operation or fitness for use or for any particular purpose of the Equipment or freedom thereof from any Liens or rights of others or any other representation or warranty whatsoever, express or implied with respect to the Equipment. | ||
| 11.10.2 | Westaim does not make, and there are no representations or warranties, express or implied, statutory or otherwise, with respect to the Equipment or this Agreement or any schedule or amendment hereto or affecting the rights of the Parties hereby and Westaim shall not be deemed to make, now or hereafter at any time, any representation or warranty, express or implied, as to the quality of the material or workmanship of the Equipment, or the conformity of the Equipment to the provisions and specifications of S&N, or to the condition, design, merchantable quality, durability, operation or fitness for use or for any particular purpose of the Equipment or freedom thereof from any Liens or rights of others or any other representation or warranty whatsoever, express or implied with respect to the Equipment. |
- 31 -
| 11.11.1 | Westaim determines in its sole discretion that additional or new equipment is required in order to carry out the terms of this Agreement (“New Equipment”), Westaim shall manufacture, or have manufactured, the New Equipment, at its sole cost. S&N shall have the option to purchase the New Equipment, or any part thereof, at any time during the six (6) months following the date Westaim advises S&N in writing that it has determined to acquire New Equipment, or at any time during the six (6) months following its decision to practice the Manufacturing License pursuant to Section 4.1, exercisable by written notice to Westaim. The purchase price for the New Equipment shall be the net book value of the New Equipment, as determined by the Parties, with such adjustments to the purchase price as necessary such that, from Westaim’s perspective, the purchase price shall have a neutral effect from a tax perspective; and | ||
| 11.11.2 | Westaim determines that it will not acquire New Equipment, S&N shall have the right to manufacture, or have manufactured, the New Equipment at its sole cost. Westaim shall cooperate with S&N by providing reasonable assistance to permit S&N to manufacture, or have manufactured, the New Equipment; provided that S&N shall reimburse Westaim for all out-of-pocket costs it incurs in providing such assistance. |
MISCELLANEOUS
| 12.1.1 | Means an event, the cause of which is beyond the reasonable control of the Party affected thereby and which could not reasonably have been foreseen and provided against, including, without limitation, acts of God, strikes, lock-outs or other labour or industrial disturbances, accidents, fires, explosions, weather conditions materially affecting or preventing work, inability to secure fuel, power, materials, contractors or labour, mechanical breakdown, failure of equipment or machinery, delays in transportation, wars, civil commotion, riot, sabotage, interruptions by government, court orders, or orders or rulings by regulatory bodies; provided that an event caused by or materially contributed to by a Party’s financial difficulty shall not be included as a force majeure event. |
- 32 -
| 12.1.2 | Notwithstanding any other provision of this Agreement, if by reason of Force Majeure, either Party is wholly or partly unable to perform certain of its obligations under this Agreement, it shall be relieved of those obligations to the extent, and for the period, that it is affected by Force Majeure, provided that the affected Party gives the other Party prompt notice of such inability and nature, cause and expected duration of the Force Majeure. The Party affected by Force Majeure shall use all reasonable efforts to remedy the situation and remove, so far as possible and with reasonable dispatch, the cause of its inability to perform, provided that there shall be no obligation on a Party so affected to settle labour disputes or to test or to refrain from testing the validity of any order, regulation or law in any court having jurisdiction. The Party affected by Force Majeure shall give prompt notice of the cessation and the cause thereof, and shall provide reports at least every seven (7) days as to its progress in dealing with the Force Majeure. Should the Force Majeure event continue for a period longer than thirty (30) business days, the Party shall no longer be relieved of its obligations under this Agreement. |
00000 Xxxxxxx Xxxx
X.X. Xxx 0000
Xxxxx, Xxxxxxx, 00000-0000
Attention: President
Facsimile: (000) 000-0000
0000 Xxxxxx Xxxx
Xxxxxxx, Xxxxxxxxx, 00000
Attention: General Counsel
Facsimile: (000)000-0000
00000—000 Xxxxxx
Xxxx Xxxxxxxxxxxx, Xxxxxxx X0X 0X0
Attention: President
Facsimile: (000) 000-0000
- 33 -
0000, 000—0xx Xxxxxx X.X.
Xxxxxxx, Xxxxxxx X0X 0X0
Attention: President
Facsimile: (000) 000-0000
Xxx Xxxxxxx Xxxx, Xxxxx 000,
Xxxxxx, Xxx Xxxxxxxxx 00000
Attention: President
Facsimile: (000)000-0000
- 34 -
- 35 -
- 36 -
- 37 -
| 12.11.1 | This Agreement shall not constitute or give rise to an agency, partnership or joint venture between the Parties and each Party’s performance hereunder is that of a separate, independent entity. | ||
| 12.11.2 | Nothing in this Agreement shall be deemed to be the grant by either Party to the other of any right, title or interest in any product, material or proprietary rights of the other except as may be expressly provided for in this Agreement. |
| Westaim Biomedical Corp. | Xxxxx & Nephew Inc. | |||||||
Per: |
/s/ Xxxxx X. Xxxxxx | Per: | /s/ Xxxxx X. Xxxxxxxx | |||||
Per:
|
/s/ [ILLEGIBLE] | Per: | /s/ Xxxxxxx Xxxx | |||||
| Xxxxx & Nephew, Inc. | ||||||||
| Per: | /s/ Xxxxx X. Xxxxxxxx | |||||||
| Per: | /s/ Xxxxxxx Xxxx | |||||||
| X.X. Xxxxx & Nephew Limited | ||||||||
| Per: | /s/ Xxxxx X. Xxxxxxxx | |||||||
| Per: | /s/ Xxxxxxx Xxxx | |||||||
 |
PRODUCT SPECIFICATION |
||||
| DESIGN PROJECT: | Acticoat Absorbent Dressing | ||||
| DHF #: | 2005 | ||||
| VERSION: | 1.2 | ||||
| 1.0 | DESIGN DESCRIPTION | |
| 1.1 | SUMMARY | |
| This Product Specification is for a calcium alginate wound dressing incorporating Acticoat technology for antimicrobial performance. The product is intended for dressing moderately to highly exudative wounds such as decubitus ulcers, diabetic ulcers and venous stasis ulcers. The product in the form of a ribbon would also be suitable for packing deep cavity wounds. Two product sizes will be produced: 4” x 5” and a 0.75” x 12” ribbon. | ||
| 1.2 | THEORY OF OPERATION OF THE PROPOSED DESIGN |
| • | Calcium alginate dressings are highly absorbent by virtue of their strong hydrophilic gel formation. | ||
| • | Calcium alginate dressings release calcium by ion exchange with sodium when contacted with wound fluids. The sodium alginate creates a viscous liquid or gel, which creates a physiologically moist microenvironment that promotes healing and the formation of granulation tissue. | ||
| • | There have been few studies of the effect of alginate wound dressings on the process of wound healing. In one study the results suggested that the calcium alginate tested may improve some cellular aspects of normal wound healing, but not others. | ||
| • | The dressing will provide antimicrobial activity through a controlled release of silver from an Acticoat coating on the alginate fibres. | ||
| • | For epithelizing wounds, the dressing can be removed without causing pain or trauma if they are first well soaked with sodium chloride solution. Residual alginate fibres trapped in a wound are readily biodegraded. | ||
| • | A secondary dressing will be required to provide attachment of this product and may provide additional absorbency for wound exudate. |
| 1.3 | DRAWINGS/SCHEMATICS (REFERENCE APPLICABLE ATTACHMENTS) | |
| Figure 1: Pouch Label for Product size 4” x 5” | ||
| Figure 2: Pouch Label for Product Size ¾” x 12” |
 |
PRODUCT SPECIFICATION |
||||
| DESIGN PROJECT: | Acticoat Absorbent Dressing | ||||
| DHF #: | 2005 | ||||
| VERSION: | 1.2 | ||||
| Figure 3: Box Label for Product size 4” x 5” | ||
| Figure 4: Box Label for Product size ¾” x 12” | ||
| Figure 5: Product Insert |
Page 2 of 18
 |
PRODUCT SPECIFICATION |
||||
| DESIGN PROJECT: | Acticoat Absorbent Dressing | ||||
| DHF #: | 2005 | ||||
| VERSION: | 1.2 | ||||
| 2.0 | COMPARISON WITH COMPETITIVE DEVICES (GOLD STANDARDS, REFERENCE STANDARDS, ETC.) |
| • | Acticoat AB for antimicrobial efficacy | ||
| • | AlgiSite M by Xxxxx & Nephew for alginate characteristics, including absorbency and gel formation. |
| 3.0 | PHYSICAL ATTRIBUTES | |
| 3.1 | MATERIALS OF CONSTRUCTION | |
| The only new material of construction required is non-woven calcium alginate. |
| 3.1.1 | Vendor/Supplier, Vendor P/N |
| • | Manufacturer: Acordis Specialty Fibers | ||
| • | Substance: Non-Sterile Needled Calcium Alginate Fabric | ||
| • | Description: Needled Alginate Fabric | ||
| • | Width: 91-95 cm. | ||
| • | Basis weight: 150 gsm ± 30 gsm |
| 3.1.2 | Rare or Single-Source Materials | ||
| The non-woven calcium alginate used for the development of this product has been procured from only one supplier. Further testing will be required to validate alternative materials from other suppliers. | |||
| 3.1.3 | Special Vendor Contract Requirement | ||
| A supply contract with Acordis Specialty Fibers has been established. The current revision is referenced in the material specification for calcium alginate (PS-0050). |
| 3.2 | CONSTRUCTION | |
| Single-ply dressing consisting of non-woven calcium alginate with Acticoat coating applied to both sides. | ||
| 3.3 | SIZES | |
| The size, as per WB-10-02-07, is acceptable if the sample falls within the dimensions below: |
| Dressing Size (on label) | Measured Dimensions, cm | |
| 4” x 5”(10 cm x 12.5 cm) | 10.2 cm (+1.2 cm, - 0.5 cm) x 12.7 cm (+1.2 cm, - 0.5 cm) |
Page 3 of 18
 |
PRODUCT SPECIFICATION |
||||
| DESIGN PROJECT: | Acticoat Absorbent Dressing | ||||
| DHF #: | 2005 | ||||
| VERSION: | 1.2 | ||||
| ¾” x 12” (2.0 cm x 30 cm) | 1.9 cm (± 0.5 cm) x 30.5 cm (+1.2 cm, - 0.5 cm) |
| 3.4 | APPEARANCE | |
| The appearance, as per WB-10-02-08, of the sample is acceptable if the following criteria are met: |
| • | both sides demonstrate a uniform colour; | ||
| • | both sides show no stripes, colour fading, patches or other discoloration; | ||
| • | clean-cut edges; and | ||
| • | no melted or burnt patches, wrinkles, rips or tears. |
| Due to the subjective nature of assessing appearance conformity, the following applies: |
| • | verify conformance to the stated specification; | ||
| • | compare the sample to work standards when possible; | ||
| • | if the observations are questionable the Quality Systems Manager will assess conformance to the specification; | ||
| • | if the product does not meet predefined requirements, reject the sample; and | ||
| • | if it is unclear whether the product meets the requirements, reject the sample and convene an MRB as per WB-13-01. |
| 3.5 | COLOUR |
| • | Due to the difficulty of measuring the product colour by the Minolta instrument, the criteria for acceptability of colour will be determined by a comparison with colour standards. |
| 4.0 | PERFORMANCE ATTRIBUTES | |
| 4.1 | PARAMETERS OF OPERATION |
| 4.1.1 | Efficacy |
| • | Will have bactericidal properties similar to Acticoat AB, by demonstrating at least a 4.0 log reduction of Pseudomonas aeruginosa in 120 minutes according to procedure WB-10-03-25; | ||
| • | Will have bactericidal properties similar to Acticoat AB, by demonstrating at least a 3 log reduction of S. aureus in 120 minutes according to procedure WB-10-03-25; | ||
| • | Will maintain antimicrobial activity for a period of three days in use, by demonstrating 3 days plate to plate CZOI efficacy using procedure WB-10-03-04. |
Page 4 of 18
 |
PRODUCT SPECIFICATION |
||||
| DESIGN PROJECT: | Acticoat Absorbent Dressing | ||||
| DHF #: | 2005 | ||||
| VERSION: | 1.2 | ||||
| 4.1.2 | Absorbency | ||
| The absorbency will be at least 12 g PBS/g dressing using procedure WB-10-03-11 | |||
| 4.1.3 | Gel Formation | ||
| The product must have gel formation properties comparable to other commercially available alginate products, by demonstrating a minimum viscosity of 2.5 cP at a shear rate of 1600 1/s, as demonstrated by procedure WB-10-03-26. | |||
| 4.1.4 | Stability | ||
| The product will have a shelf life of at least one year from the date of manufacture as established by accelerated aging studies according to the method outlined in Westaim Biomedical Report #000331. |
| 5.0 | SAFETY ATTRIBUTES | |
| 5.1 | BIOCOMPATABILITY |
| • | Biocompatible as determined by cytotoxicity, sensitization and irritation tests |
| 5.2 | KNOWN LIMITATIONS (CAUTIONS) |
| • | for external use only; | ||
| • | do not use on patients with a known sensitivity to silver; | ||
| • | Should not be used on dry or non-exuding wounds; | ||
| • | May cause transient discoloration; | ||
| • | not compatible with oil-based products such as petrolatum; | ||
| • | not compatible with MRI (Magnetic Resonance Imaging) procedures; | ||
| • | not compatible with ECG procedures; | ||
| • | avoid exposure to temperatures above 120°F (50°C); | ||
| • | single use only; | ||
| • | do not re-sterilize; | ||
| • | do not use if package is damaged |
| 5.3 | HUMAN FACTORS CONSIDERATIONS |
| • | Instructions for use will be provided on a package insert Training will be available. | ||
| • | Intended for use by professional health care providers. |
Page 5 of 18
 |
PRODUCT SPECIFICATION |
||||
| DESIGN PROJECT: | Acticoat Absorbent Dressing | ||||
| DHF #: | 2005 | ||||
| VERSION: | 1.2 | ||||
| • | Not intended for OTC sales. | ||
| • | The dressing should be non-directional or the directionality should be clearly indicated to prevent the dressing from being applied with the wrong side to the wound. |
| • | Cleanse the wound using conventional, non oil-based techniques and leave the wound moist. | ||
| • | Remove Acticoat™ Absorbent Dressing from the package. | ||
| • | Apply the dressing to the wound and secure with an appropriate secondary dressing that will maintain a moist environment. | ||
| • | Ensure the dressing remains moist, but not so wet that tissue maceration occurs. | ||
| • | Change the dressing depending on the amount of exudate and the condition of the wound. | ||
| • | If the dressing dries and adheres to the wound, moisten or soak the dressing prior to removal. | ||
| • | Avoid forceful removal and disruption of the healing wound. |
| 5.4.1 | Equipment/Materials Required for Use of the Device |
| • | Scissors | ||
| • | Appropriate secondary dressing | ||
| • | Adhesive tape or other attachment products |
| 6.0 | STERILIZATION | |
| 6.1 | Sterilization Method: E-beam irradiation | |
| 6.2 | Sterilization Verification: Validation testing to achieve a XXX of 10-6. | |
| 6.3 | Sterilization Special Concerns: Irradiation should not exceed 50 kGy for a single dose or 75 kGy for a total dose. | |
| 7.0 | PACKAGING | |
| 7.1 | PACKAGING CONFIGURATIONS | |
| The product will be produced in two sizes: 4” x 5” and ¾” x 12” that will be packaged in peelable pouches 6” x 8 ½” and 3” x 8” respectively. The ¾” x 12” dressing will be folded two times along it’s length, ¾” x 4”, prior to packaging. The pouches will be packaged 5 per box. Boxes will packed in plain cardboard cases for shipping. |
Page 6 of 18

|
PRODUCT SPECIFICATION | |||||
| DESIGN PROJECT : | Acticoat Absorbent Dressing | |||||
| DHF #: | 2005 | |||||
| VERSION : | 1.2 | |||||
| Part number: | 4” x 5” Front: A3082 Back: A3000 | |||
| ¾” x 12” Front: A3080 Back: A3007 | ||||
| Composition: | Front: Tyvek 1073B (Spunbonded polyolefin) coated with SBP 2000 and printed. | |||
| Back: Tyvek 1073B (Spunbonded polyolefin) uncoated and unprinted. | ||||
| Basis Weight: | 2.57 ± 0.21 oz./sq. yd. (8.71 ± 0.71 mg/cm2) | |||
| Roll Widths: | 3” ± 0.25”, 6” ± 0.25” | |||
| Appearance: | Clean, white, and no wrinkles or tears. | |||
| Printing: | The images shown in Figures 1 & 2, are printed on the coated side of the Tyvek. The printed side should be on the outside of the roll with the top of the label facing the leader end of the roll. | |||
| Colours: | The Pantone colours used for printing are as follows: | |||
| Blue 286 | ||||
| Grey Cool Grey 6 | ||||
| Black | ||||
| Certification: | Technical specifications must be certified for each lot of Tyvek packaging. Supplier quality documentation that includes composition, product type, part identification (lot number), and quantity shipped must accompany each shipment. | |||
| Contents: | Peelable pouch with chevron seal, each containing one dressing. | |||
| Dimensions: | For 4” x 5” product: 153 mm x 216 mm (6” x 8 ½”) | |||
| For ¾” x 12” product 76.2 mm x 204 mm (3” x 8”) | ||||
| Size tolerance ± 6.35 mm (± 0.25”) | ||||
| Label: | The label appearance is acceptable as per WB- 10-02-03 if the following criteria are met: | |||
| a) the label is centered on the package; | ||||
| b) the lot and expiry date stamps are straight and in the bottom left xxxxx; | ||||
| c) the printing is readable; and | ||||
| d) the label matches the dressing in the package. | ||||
| Seal: | The seal size and position, as per WB- 10-02-04, is acceptable if the following criteria are met: |

|
PRODUCT SPECIFICATION | |||||
| DESIGN PROJECT : | Acticoat Absorbent Dressing | |||||
| DHF #: | 2005 | |||||
| VERSION : | 1.2 | |||||
| a) the seal is at least 7 mm wide, within 7 mm from the side edge and within 10 mm from the bottom edge; and | ||||
| b) the Chevron seal is at the top of the label. | ||||
| Seal Integrity: | A continuous seal with no bubbles, blisters or other inconsistencies as per WB-10-02-06 and WB-10-02-02. | |||
| If channels are observed during the dye penetration test, a minimum seal width of 7 mm must be maintained to accept the results. | ||||
| Seal Strength: | All package pouches will have a burst test pressure of at least 1.0 psig as per WB-10-02-05. |
| 7.1.2 | Outer Packaging (Box) |
| Part number: | 4”x5”:A4082 | |||
| ¾”xl2”:A4080 | ||||
| Composition: | Cardboard, 0.02 clay coated solid bleached sulphite with 1 mil poly coated backside; | |||
| Printing: | Printed with images shown in Figure 3 & 4; | |||
| Colours: | The Pantone colours used for printing are as follows: | |||
| Blue 286 | ||||
| Grey Cool Grey 6 | ||||
| Black | ||||
| Appearance: | White, no wrinkles, straight folds, side glued with tuck ends. | |||
| Box style: | Notched reverse tuck end (French) carton. | |||
| Contents: | box only: 5 dressings/box; | |||
| optional bag & box configuration: 5 dressings/bag, 1 bag/box. | ||||
| Pouches shall be oriented with the label facing up towards the opening of the box and with the label oriented in the same direction as the box label. | ||||
| Dimensions: | For 4” x 5” product: | |||
| 232 mm x 156 mm x 19 mm (8 5/8 “ x 6 1/8” x 3/4”) | ||||
| For ¾ ” x 12” product: | ||||
| 206 mm x 80 mm x 25 mm (8 1/8” x 3 1/8” x 1”) | ||||
| Inserts: | Each box contains one product insert. |

|
PRODUCT SPECIFICATION | |||||
| DESIGN PROJECT : | Acticoat Absorbent Dressing | |||||
| DHF #: | 2005 | |||||
| VERSION : | 1.2 | |||||
| Labeling: | Each box shall be labeled with a product code and size and lot/expiry date as defined on the Boxing and Casing Operating Specification. | |||
| One label is affixed to the bottom end of each box. |
| 7.1.3 | Outer Packaging (Case) | |
| Unprinted cases, the same as those used for the Acticoat Burn Dressing, will be used for shipping bulk Acticoat Alginate Dressings. | ||
| 7.1.4 | Product Insert |
| Part number: | A5008 | |||
| Composition: | Hammermill Bond/wr 24 lb., white paper | |||
| Dimensions: | 5.5” x 8.5” (14.0 cm x 21.6 cm) folded into 3 sections, with the printed side facing out, to make a 5.5” x 2.83” insert | |||
| Appearance: | Clean, white, and no wrinkles or tears. | |||
| Printing: | Printed with the image shown in Figure 5. | |||
| Colours: | The Pantone colours used for printing are as follows: | |||
| Grey 38% of Black | ||||
| Black |
| 8.0 | LABELING | |
| 8.1 | LANGUAGE REQUIREMENT |
| • | English, French-Canadian, Latin American Spanish |
| 8.2 | PACKAGE LABELS |
| • | See Figures 1-5 at end of document |
| 9.0 | REGULATORY COMPLIANCE |
| • | US FDA 510(k) AND HPB notification; | ||
| • | MDD compliance |
| 10.0 | MANUFACTURING/QC METHODS | |
| 10.1 | KEY PROCESSES |

|
PRODUCT SPECIFICATION | |||||
| DESIGN PROJECT : | Acticoat Absorbent Dressing | |||||
| DHF #: | 2005 | |||||
| VERSION : | 1.2 | |||||
| • | The coating of silver onto the non-woven calcium alginate will be carried out using a similar process as that used for coating HDPE. The roll of alginate will be fitted with a leader and trailer of tyvek to minimize waste of alginate in the start up and finish stages of the roll to roll coating. Coating of the alginate will be conducted in four passes, two for each side (1 basecoat pass & 1 topcoat pass). | ||
| • | Slitting of the alginate web will be conducted using a crush cutting module. | ||
| • | Packaging will be performed using the TM Packaging Machine. | ||
| • | An approved contractor will conduct sterilization of pouches. Packing of pouches into boxes and cases will be carried out after sterilization. |
| • | Slitting and packaging will be performed in the controlled environment room. |
| 10.3 | APPLICABLE QUALITY CONTROL STANDARDS | |
| The product will be manufactured in accordance with the quality practices specified in the following documents: |
| • | EN 46001:1996, Quality Systems — Medical Devices — Particular Requirements for the Application of EN29001 | ||
| • | ISO 13485:1996, Quality Systems — Medical Devices — Particular Requirements for the Application of ISO 9001 | ||
| • | Quality System Regulations specified in the Current Good Manufacturing Practices (CGMP) for medical devices; 21 CFR part 820 |
| 10.4 | PROPOSED QUALITY CONTROL TESTING / TEST REQUIREMENTS |
| 10.4.1 | Raw Materials Testing: Non-woven calcium alginate: | ||
| The width, thickness and basis weight will be confirmed during the material receiving and according to procedure WB-10-01; | |||
| The non-woven calcium alginate requires validation as a raw material. Until validation is completed, each lot of material received will require a subsequent product validation. |
| 10.4.2 | In-Process Testing: | ||
| Both ends of each roll of silver coated alginate will be tested for total silver and NH4OH soluble silver before slitting. Three samples will be taken to represent the left, centre and right sections of the material according to WB-09-03-02. |

|
PRODUCT SPECIFICATION | |||||
| DESIGN PROJECT : | Acticoat Absorbent Dressing | |||||
| DHF #: | 2005 | |||||
| VERSION : | 1.2 | |||||
| Characteristic | Procedure | Specification | ||
Total Silver
|
WB-10-02-14 | 0.88 mg/cm2 – 1.40 mg/cm2 | ||
NH4OH soluble silver
|
WB-10-02-14 | ³ 0.45 mg/cm2 providing that it is between 35% and 80% of the Total Silver |
| 10.4.3 | Finished Device Testing: | |
| The finished product must have the following performance characteristics: |
| Characteristic | Procedure | Specification | ||
Size
|
WB-10-02-07 | As section 3.3 | ||
Appearance
|
WB-10-02-08 | As section 3.4 | ||
NH4OH soluble silver (at the
time of release)
|
WB-10-02-14 | ³ 0.45 mg/cm2 providing that it is between 35% and 80% of the Total Silver |
||
NH4OH soluble silver (at the
end of the shelf life)
|
WB-10-02-14 | ³ 0.20 mg/cm2 providing it is greater than 20% of the Total Silver | ||
Total Silver
|
WB-10-02-14 | 0.88 mg/cm2 – 1.40 mg/cm2 | ||
Pouch label appearance
|
WB-10-02-03 | As per section 7.1.1 | ||
Pouch seal size & position
|
WB-10-02-04 | As per section 7.1.1 | ||
Pouch seal strength
|
WB-10-02-05 | As per section 7.1.1 | ||
Pouch seal integrity
|
WB-10-02-02 | As per section 7.1.1 | ||
| WB-10-02-06 |
| 10.5 | EQUIPMENT TO BE VALIDATED: | |
| Crush Cutting Nodule | ||
| 10.6 | PROCESSES TO BE VALIDATED: | |
| Coating, slitting, packaging, sterilization, burst testing and silver analysis. |

|
PRODUCT SPECIFICATION | |||||
| DESIGN PROJECT : | Acticoat Absorbent Dressing | |||||
| DHF #: | 2005 | |||||
| VERSION : | 1.2 | |||||
| 11.0 | Shelf Life |
| 12.0 | Approval |
| Department | Name/Signature | Title | Date | |||
Project Leader |
||||||
Marketing |
||||||
Science and
Technology |
||||||
Manufacturing |
||||||
Quality Assurance |
||||||
Regulatory Affairs |
||||||
New Product Development |

|
DESIGN PROJECT: | PRODUCT SPECIFICATION Acticoat Absorbent Dressing |
||||
| DHF #: | 2005 | |||||
| VERSION: | 1.2 | |||||

Page 13 of 18

|
DESIGN PROJECT: | PRODUCT SPECIFICATION Acticoat Absorbent Dressing |
||||
| DHF #: | 2005 | |||||
| VERSION: | 1.2 | |||||

Page 14 of 18

|
DESIGN PROJECT: | PRODUCT SPECIFICATION Acticoat Absorbent Dressing |
||||
| DHF #: | 2005 | |||||
| VERSION: | 1.2 | |||||

Page 15 of 18

|
DESIGN PROJECT: | PRODUCT SPECIFICATION Acticoat Absorbent Dressing |
||||
| DHF #: | 2005 | |||||
| VERSION: | 1.2 | |||||

Page 16 of 18

|
DESIGN PROJECT: | PRODUCT SPECIFICATION Acticoat Absorbent Dressing |
||||
| DHF #: | 2005 | |||||
| VERSION: | 1.2 | |||||

Page 17 of 18

|
DESIGN PROJECT: | PRODUCT SPECIFICATION Acticoat Absorbent Dressing |
||||
| DHF #: | 2005 | |||||
| VERSION: | 1.2 | |||||
Page 18 of 18
Westaim Biomedical Corp, Xxxxx & Nephew Inc, Xxxxx &
Nephew, Inc. and X. X. Xxxxx & Nephew Limited

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| 1.0 | DESIGN DESCRIPTION | |
| 1.1 | SUMMARY |
| 1.1.1 | This specification is for the Acticoat Moisture Control Dressing, that is intended to provide superior performance by extending the duration of use to seven days and by combining antimicrobial activity with a polyurethane foam that provides a light to moderate amount of absorbency. One product size will be produced: 5” x 5” (Product code: 20211). |
| 1.2.1 | Foam will be used as the absorbent to enhance antimicrobial activity of the silver coating by providing a zone, and residence time, for killing of microorganisms. | ||
| 1.2.2 | The foam in sufficient thickness will be used to absorb a specified amount of wound exudate. | ||
| 1.2.3 | The dressing will provide antimicrobial activity through a sustained release of silver, which will minimize bacterial penetration to the wound and within the dressing. | ||
| 1.2.4 | To maintain a moist wound environment, an appropriate secondary dressing may be necessary to limit moisture evaporation. |
| 1.3 | DRAWINGS/SCHEMATICS (REFERENCE APPLICABLE ATTACHMENTS) |
| 1.3.1 | The following drawings and schematics are shown at the end of the document: |
| • | Figure 1: Package Label | ||
| • | Figure 2: Box Label | ||
| • | Figure 3: Product Insert |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| 2.0 | COMPARISON WITH COMPETITIVE DEVICES | |
| 2.1 | The gold standards for this product are the Acticoat® silver-coated 5 layer Foam Dressing (FDA Number — K000051, Westaim Biomedical Corp.) in terms of antimicrobial activity, and Hydrasorb Sponge Dressing (Avitar Inc.) in terms of physical characteristics. | |
| 3.0 | PHYSICAL ATTRIBUTES | |
| 3.1 | MATERIALS OF CONSTRUCTION |
| 3.1.1 | New Materials | ||
| The only new material of construction required is hydrophilic polyurethane foam. The Westaim part number is A2005. | |||
| 3.1.2 | Vendor/Supplier Information |
| Manufacturer: | Xxxxxxx Manufacturing Inc. | |||
| Substance: | Hydrophilic polyurethane foam | |||
| Description: | Medical grade TIGHT CELL foam | |||
| Width: | As indicated on the Purchase Order | |||
| Thickness: | 1/4” ± 1/1 6” (0.64 ± 0.16 cm) | |||
| Absorbency: | 15.0 ± 4.0 g of water / g of foam | |||
| Density: | 9.1 ± 0.8 g/100 cm3 |
| 3.1.3 | Rare or Single-Source Materials | ||
| The hydrophilic polyurethane foam used for the development of this product has been procured from only one supplier. Further testing will be required to validate alternative materials from other suppliers. | |||
| 3.1.4 | Special Vendor Contract Requirement |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| Xxxxxxx Manufacturing Inc. will be provided with the current raw material specification. The current specification (PS-0056) will be included with, or referenced on each purchase order. |
| 3.2 | CONSTRUCTION |
| 3.2.1 | One-ply hydrophilic polyurethane foam coated with Acticoat nanocrystalline silver on both sides. |
| 3.3 | SIZES |
| 3.3.1 | The size, as per WB-10-02-07, is acceptable if the sample falls within the dimensions defined in the table below. |
| Dressing Size (on label) | Measured Dimensions (cm) | |
5”
× 5”
|
Length - 12.7 (+ 1.2, - 0.5) | |
(12.5
cm × 12.5 cm)
|
Width - 12.7 (+1.2, - 0.5) | |
| Thickness - 0.64 (+ 0.16, - 0.32) |
| 3.4 | APPEARANCE |
| 3.4.1 The appearance, as per WB-10-02-08, of the sample is acceptable if the following criteria are met: |
| • | both sides demonstrate a uniform color when compared to the appearance standards as per WB-10-02-31 | ||
| • | the presence of white spots does not exceed the approved standards | ||
| • | both sides show no strong stripes, creases, colour fading, patches or other discoloration | ||
| • | the four edges are clean-cut with two edges open and two edges closed | ||
| • | only minor pinching or separation is observed along the open or closed sides | ||
| • | no melted or burnt patches, wrinkles, rips or tears are present |
| 3.4.2 | Due to the subjective nature of assessing appearance conformity, the following applies: |
| • | verify conformance to the stated specification |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| • | compare the sample to work standards when possible | ||
| • | if the observations are questionable the Quality Systems Manager will assess conformance to the specification | ||
| • | if the product does not meet predefined requirements, reject the sample | ||
| • | if it is unclear whether the product meets the requirements, reject the sample, have the product quarantined as per WB-15-02-10, and convene an MRB as per WB-13-01 |
| 3.5 | COLOR |
| 3.5.1 | The product will be a uniform xxxx color. | ||
| 3.5.2 | Due to the difficulty of measuring the product colour by the Minolta instrument, the acceptability of color will be determined by comparison to a set of colour standards. |
| 4.0 | PERFORMANCE ATTRIBUTES | |
| 4.1 | PARAMETERS OF OPERATION |
| 4.1.1 | Efficacy |
| • | Will produce at least a 4.0 log reduction of Pseudomonas aeruginosa and a 3.0 log reduction of Staphylococcus aureus in 120 minutes according to procedure WB-10-03-24. | ||
| • | Will maintain antimicrobial activity by demonstrating 7 days plate to plate CZOI efficacy using procedure WB-10-03-04. |
| 4.1.2 | Absorbency |
| • | The absorbency without compression will be at least 12.0 g of water / g of dressing using procedure WB-10-03-11. | ||
| • | The absorbency under equivalence of 50 mmHg pressure will be at least 10.0 g of water/g of dressing using procedure WB-10-03-27. |
| 4.1.3 | Stability | ||
| The shelf life of the product should be two years from the date of coating. However, at the time of initial launch, a six month shelf life will be specified until sufficient data is established to justify a shelf life of two years. |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| 4.2 | EQUIPMENT/MATERIALS |
| 4.2.1 | The following equipment is required for use of the device: |
| • | scissors | ||
| • | appropriate secondary dressing | ||
| • | adhesive tape or other attachment products |
| 4.3.1 | The following directions for use will be specified: |
| • | cleanse the wound using conventional, non-oil based techniques and leave wound moist | ||
| • | remove the dressing from the package | ||
| • | apply the dressing to the wound and secure with an appropriate secondary dressing that will maintain a moist environment | ||
| • | ensure that the dressing remains moist, but not so wet that tissue maceration occurs | ||
| • | change the dressing depending on the amount of exudate and the condition of the wound | ||
| • | if the dressing dries and adheres to the wound, moisten or soak the dressing prior to removal | ||
| • | avoid forceful removal and disruption of the healing wound |
| 5.0 | VOLUNTARY STANDARDS |
| 5.1 | Voluntary standards include bioactivity, antimicrobial longevity and absorbency standards as specified in section 4.1. |
| 6.1 | BIOCOMPATIBILITY |
| 6.1.1 | Biocompatible as determined by cytotoxicity (biological reactivity), dermal irritation, skin sensitization and porcine model wound healing tests. |
| 6.2 | KNOWN LIMITATIONS (CAUTIONS) |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| 6.2.1 | The following known limitations will be specified: |
| • | for external use only | ||
| • | should not be used on patients with a known sensitivity to silver | ||
| • | do not use on dry or non-exuding wounds unless the dressing is pre-moistened | ||
| • | may cause transient discoloration | ||
| • | not compatible with oil-based products, such as petrolatum | ||
| • | not compatible with MRI (Magnetic Resonance Imaging) procedures | ||
| • | should not come in contact with electrodes or conductive gels during electronic measurements | ||
| • | avoid exposure to temperatures above 120°F (50°C) | ||
| • | do not use if product color is not uniform | ||
| • | single use only | ||
| • | do not re-sterilize | ||
| • | do not use if package is damaged |
| 7.0 | HUMAN FACTORS | |
| 7.1 | Instruction for use will be provided on a package insert. Training will be available. | |
| 7.2 | The product is intended for use by professional health care providers only. | |
| 7.3 | The product is not intended for OTC sales. | |
| 7.4 | The dressing should be non-directional, or the directionality should be clearly indicated to prevent the dressing from being applied with wrong side to the wound. | |
| 8.0 | STERILIZATION | |
| 8.1 | STERILIZATION METHOD |
| 8.1.1 | The product will be sterilized using electron beam irradiation. |
| 8.2 | STERILIZATION VERIFICATION |
| 8.2.1 | The adequacy of the sterilization processes will be assessed by demonstrating that a Sterility Assurance Level (XXX) of 10-6 is achieved. |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| 8.3 | SPECIAL CONCERNS |
| 8.3.1 | Not applicable. |
| 9.0 | PACKAGING | |
| 9.1 | PACKAGING CONFIGURATION |
| 9.1.1 | The product will be produced in one size: 5” x 5” that will be packaged in a 7” x 10” peelable pouch. The pouches will be packaged 5 per box and the boxes will be contained in plain cardboard cases. |
| 9.2 | PACKAGING MATERIALS |
| 9.2.1 | Primary Packaging (pouch) |
| Part number: | 5” x 5” Front – P/N A3041 Back — P/N A3008 | |||
| Composition: | Front – Tyvek 1073B (Spunbonded polyolefin) coated with SBP 2000
and printed Back – Tyvek 1073B (Spunbonded polyolefin) uncoated and imprinted |
|||
| Basis Weight: | 2.57 ± 0.21 oz./sq.yd. (8.71 ± 0.71 mg/cm2) | |||
| Roll Widths: | 7” ± 0.25” | |||
| Appearance: | Clean, opaque, white, and no wrinkles or tears | |||
| Printing: | The image shown in Figure 1 will be printed on the coated side of the Tyvek. The printed side will be on the outside of the roll with the top of the label facing the leader end of the roll. | |||
| Colors: | The Pantone colors used for printing are as follows: | |||
| • Blue 286 | ||||
| • Grey Cool Grey 6 | ||||
| • Green 3415 |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| • | Black | |||||
| Certification: | Technical specifications will be certified for each lot of Tyvek packaging. Supplier quality documentation that includes composition, product type, part identification (lot number), and quantity shipped will be required with each return shipment. | |||||
| Contents: | Peelable pouch with chevron seal, each containing one dressing. | |||||
| Dimensions: | The 5” x 5” product will be 178 mm wide x 254 mm long (7” x 10”) with a size tolerance ± 6.35 mm (± 0.25”) | |||||
| Label: | The label appearance will be acceptable as per WB-10-02-03 if the following criteria are met: | |||||
| • | the label is centered on the package | |||||
| • | except for the internal part number for the packaging material, printing should not carry over into the seam | |||||
| • | the lot and expiry date stamps are straight and in the bottom left corner | |||||
| • | the printing is readable | |||||
| • | the label matches the dressing in the package | |||||
| Seal: | The seal size and position will be acceptable as per WB-10-02-04 if the following criteria are met: | |||||
| • | the seal is at least 7 mm wide, within 7 mm from side edge and within 10 mm from the bottom edge | |||||
| • | the Chevron seal is at the top of the label | |||||
| Seal Integrity: | The seal integrity will be acceptable as per WB-10-02-06 and WB-10-02-02 if the seal is continuous with no bubbles, blisters or other inconsistencies that impact package integrity. If channels are observed during the dye penetration test, a minimum seal width of 7 mm must be maintained to accept the results. | |||||

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| Seal Strength: | All package pouches will be subject to a burst test. The burst pressure as per WB-10-02-05 will be acceptable if it is at least l.0 psig. |
| 9.2.1 | Outer Packaging (box) |
| Part number: | 5” x 5”: A4041 | |||
| Composition: | Cardboard, 0.02 clay coated solid bleached sulfite with 0.5 mil poly coated backside | |||
| Printing: | Printed with the images shown in Figure 2 | |||
| Colors: | The Pantone colors used for printing are as follows: | |||
| • Blue 286 | ||||
| • Grey Cool Grey 6 | ||||
| • Green 3415 | ||||
| • Black | ||||
| Appearance: | White, no wrinkles, straight folds, side glued with tuck ends | |||
| Box Style: | Notched reverse tuck end (French) carton | |||
| Contents: | 5 dressings/box. Pouches will be oriented with the label facing up towards the opening of the box and with the label oriented in the same direction as the box label. | |||
| Dimensions: | The 5” x 5” product will be 181 mm wide x 257 mm long x 32 mm thick (7 1/8” x 10 1/ 8” x 1 1/4”) | |||
| Inserts: | Each box will contain one product insert | |||
| Labeling: | Each box will be labeled with a lot/expiry date as defined on the Boxing and Casing Operating Specification. One label will be affixed to the bottom end of each box. |
| 9.2.3 | Outer Packaging (Case) |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| Unprinted cases, the same as those used for the Acticoat Burn Dressing, will be used for shipping bulk Acticoat Moisture Control Dressings. | |||
| 9.2.4 | Product Insert |
| Part number: | A5003 | |||
| Composition | Paper — Hammermill Bond/wr 24 lb., white paper | |||
| Dimensions: | 5.5” x 8.5” folded into 3 sections to make 5.5” x 2.83” | |||
| Appearance: | Clean, white, and no wrinkles or tears | |||
| Printing: | Printed with the image shown in Figure 3 | |||
| Colors: | The Pantone colors used for printing are as follows: | |||
| • Green 3415 | ||||
| • Black |
| 10.0 | LABELLING | |
| 10.1 | Language Requirement: English, French-Canadian and Latin American Spanish. | |
| 10.2 | Package Labels: see Figures 1-3 at the end of this document. | |
| 11.0 | REGULATORY COMPLIANCE | |
| 11.1 | The product will be developed, manufactured and distributed in accordance with the legal requirements in the jurisdiction in which the product is to be sold. | |
| 11.2 | Notification: US FDA via 510(k), HPB and Notified Body. | |
| 11.3 | The product will be developed, manufactured and distributed in accordance with the requirements stated in the MDD. | |
| 12.0 | QUALITY STANDARDS |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| 12.1 | The product will be developed, manufactured and distributed in accordance with the quality practices specified in the following documents: |
| • | EN 46001:1996, Quality Systems — Medical Devices — Particular Requirements for the Application of EN 29001 | ||
| • | ISO 13485:1996, Quality Systems — Medical Devices — Particular Requirements for the Application of ISO 9001 | ||
| • | Quality System Regulations specified in the Current Good Manufacturing Practices (CGMP) for medical devices; 21 CFR part 820 |
| 13.0 | MANUFACTURING | |
| 13.1 | KEY PROCESSES |
| 13.1.1 | Not applicable. |
| 13.2 | ENVIRONMENTAL REQUIREMENTS |
| 13.2.1 | Not applicable. |
| 13.3 | NEW EQUIPMENT TO BE VALIDATED |
| 13.3.1 | Not applicable. |
| 13.4 | NEW PROCESSES TO BE VALIDATED |
| 13.4.1 | Post sterilization pumping process. |
| 14.0 | QUALITY ASSURANCE |
| 14.1 | EQUIPMENT TO BE VALIDATED |
| 14.1.1 | Not applicable. |
| 14.2 | PROCESSES TO BE VALIDATED |
| 14.1.2 | Not applicable. |
| 14.3 | QUALITY TESTING |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| 14.3.1 | Hydrophilic Polyurethane Foam |
| • | A certificate of analysis is required from the vendor for width, thickness, density tensile strength, and absorbency. | ||
| • | The thickness, width of the rolls and the basis weight will be confirmed during the material receiving and according to procedure WB-10-01. The vendor will provide certification for the other technical specifications. | ||
| • | The hydrophilic polyurethane foam requires validation as a raw material. Until validation is completed, each lot of material received will require a subsequent validation of the finished product. |
| 14.3.2 | In-Process Testing: | ||
| Both ends of each roll of silver-coated foam will be tested for total silver and NH4OH soluble silver. Three samples will be taken to represent the left, center and right sections of the material according to WB-09-03-02. The silver content must meet the requirements specified in the table below. |
| Characteristic | Procedure | Specification | ||
Total Silver
|
WB-10-02-14 | 0.88–1.50 mg/cm2 | ||
NH4OH Soluble
Silver
|
WB-10-02-31 | ³0.45 mg/cm2, providing that it is between 35% to 80% of the total silver |
| 14.3.3 | Finished Device Testing: | ||
| The finished product must have the performance characteristics specified in the table below. |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| Characteristic | Procedure | Specification | ||
Size
|
WB-10-02-07 | As section 3.2 | ||
Appearance
|
WB-10-02-08 | As section 3.3 | ||
Colour
|
WB-10-02-31 | Comparison of product to standard colour specimens. | ||
Total silver
|
WB-10-02-14 | 0.88-1.50 mg/cm2 | ||
NH4OH soluble silver
|
WB-10-02-30 | ³0.45 mg/cm2 providing that it is between 35% and 80% of the total silver at the time of release. | ||
NH4OH soluble silver
|
WB-10-02-30 | ³0.25 mg/cm2 providing it is greater than 20% of the total silver at the end of shelf life. | ||
Pouch label appearance
|
WB-10-02-03 | As section 9.2.1 | ||
Pouch seal size & position |
WB-10-02-04 | As section 9.2.1 | ||
Pouch seal strength
|
WB- 10-02-05 | As section 9.2.1 | ||
Pouch seal integrity
|
WB-10-02-02 | As section 9.2.1 | ||
| WB-10-02-06 |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |
| 15.0 | SHELF LIFE |
| 16.0 | APPROVAL |
| Department | Name/Signature | Title | Date | |||||||||
Project Leader |
||||||||||||
Marketing |
||||||||||||
Science and
Technology |
||||||||||||
Manufacturing |
||||||||||||
Quality
Assurance |
||||||||||||
Regulatory
Affairs |
||||||||||||
New Product
Development |
||||||||||||

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |


|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |


|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |

|
PRODUCT SPECIFICATION |
| DESIGN PROJECT: | Acticoat Moisture Control Dressing |
|||
| DHF#: | 2006 |
|||
| VERSION: | 1.2 |

Westaim Biomedical Corp, Xxxxx & Nephew Inc, Xxxxx &
Nephew, Inc. and X. X. Xxxxx & Nephew Limited

|
PRODUCT SPECIFICATIONS |
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
| 1. | DESIGN DESCRIPTION | |
| 1.0 | SUMMARY | |
| This document provides the specifications for the Acticoat® Burn Dressing. Five product configurations have been developed: 4” x 4” (Part #: 20101 D, B or C) and 4” x 8” (Part #: 20201 D, B or C), 8” x 16” (Part #: 20301 D, B or C), 16” x 16” (Part #: 20401 D, B or C), and 4”x48” roll (Part #:20501 D, B, C) | ||
| 1.1 | THEORY OF OPERATION |
| • | To provide 2-3 days of usable antimicrobial performance in a silver-coated wound dressing. | ||
| • | The dressing will provide antimicrobial activity through a sustained release of silver, which will minimize bacterial penetration to the wound and within the dressing. |
| 1.2 | DRAWINGS/SCHEMATICS (REFERENCE APPLICABLE ATTACHMENTS) | |
| Figure 1: Package Label (4” x 4”) | ||
| Figure 2: Box Label (4” x 4”) | ||
| Figure 3: Case Label (4” x 4”) | ||
| Figure 4: Product Insert |
| 1.3 | MATERIALS OF CONSTRUCTION |
| 1.3.1 | The materials used in the construction of the Acticoat Burn Dressing are: |
70/30% Rayon/polyester gauze — Part number: A2000
Silver targets — Part number: A0100
| Specifications for these materials are located in the QSI Specification Control database. Vendors approved to supply the different raw materials for the burn dressing are contained within Westaim Biomedical’s Approved Supplier List. |

|
PRODUCT SPECIFICATIONS |
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |

|
PRODUCT SPECIFICATIONS |
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
| Appearance: | White, no wrinkles, straight folds, side glued with tuck ends. |
| C. | OUTER PACKAGING (CASE) |
| Composition: | Corrugated case Kraft 175B, silver, outside regular adhesive. | |||
| Printing: | None (An adhesive label will be affixed to each end of the box) | |||
| See Figure 3 for the 4” x 4” label. | ||||
| Colours: | The Pantone colours used for printing the label are as follows: | |||
| Blue 286 | ||||
| Grey Cool Grey 6 | ||||
| Black | ||||
| Appearance: | White exterior, no wrinkles, straight folds, side glued with tuck ends. |
| D. | PRODUCT INSERT |
| Composition: | Paper — Hammermill Bond/wr 24 lb., white paper | |||
| Dimensions: | 5.5” x 8.5” (14.0 cm x 21.6 cm) folded into 3 sections, with the printed side facing out, to make a 5.5” x 2.83” insert. | |||
| Printing: | The label is printed in 3 languages and shown in Figure 4 | |||
| Colours: | The Pantone colours used for printing are as follows: | |||
| Blue 286 | ||||
| Black | ||||
| Appearance: | Clean, white, and no wrinkles or tears. |
| 1.4.3. | PACKAGING SPECIFICATIONS |
| A. | PRIMARY PACKAGING (POUCH) |
| Contents: | Peelable pouch with chevron seal, each containing one dressing. | |||||
| Dimensions: | For 4” x 4” product: | 152 mm x 203 mm (6” x 8”) | ||||
| For 4” x 8” product: | 152 mm x 305 mm (6” x 12”) | |||||
| For 8” x 16” product: | 254 mm x 305 mm (10” x 12”) | |||||
| For 16” x 16” product: | 254 mm x 305 mm (10” x 12”) | |||||
| For 4” x 48” product: | 152 mm x 254 mm (6” x 10”) | |||||
| Size tolerance ± 6.35 mm (± 0.25”). | ||||||

|
PRODUCT SPECIFICATIONS |
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
| Label: | The label appearance is acceptable as per WB-10-02-03 if the following criteria are met: | |||||
| a) | the label is centered on the package; | |||||
| b) | except for the internal part number for the packaging material, printing must not carry over into the seam; | |||||
| c) | the lot and expiry date stamps are straight and in the bottom left corner; | |||||
| d) | the printing is readable; and | |||||
| e) | the label matches the dressing in the package. | |||||
| Seal: | The seal size and position, as per WB-10-02-04, is acceptable if the following criteria are met: | |||||
| a) | the seal is at least 7 mm wide, within 7 mm from the side edge and within 10 mm from the bottom edge; and | |||||
| b) | the Chevron seal is at the top of the label. | |||||
| Seal Integrity: | A continuous seal with no bubbles, blisters or other inconsistencies as per WB-10-02-06 and WB-10-02-02. | |||||
| If channels are observed during the dye penetration test, a minimum seal width of 7 mm must be maintained to accept the results. | ||||||
| Seal Strength: | All package pouches will have a burst test pressure of at least 1.0 psig as per WB-10-02-05. | |||||
| B. | OUTER PACKAGING (BOX) |
| Box style: | Notched reverse tuck end (French) carton. | |||
| Contents: | Each box contains 6 or 12 dressings as indicated on the label. | |||
| Pouches shall be oriented with the label facing up towards the opening of the box and with the label oriented in the same direction as the box label. | ||||
| Dimensions: | For 4” x 4” product: | |||
| 156 mm x 206 mm x 25 mm (6 1/8” x 8 1/8” x 1”) | ||||
| For 4” x 8” product: | ||||
| 156 mm x 308 mm x 25 mm (6 1/8” x 12 1/8” x 1”) | ||||
| For 8” x 16” product: | ||||
| 257 mm x 308 mm x 25 mm (10 1/8” x 12 1/8” x 1”) | ||||
| For 16” x 16” product: | ||||
| 257 mm x 308 mm x 25 mm (10 1/8” x 12 1/8” x 1”) |

|
PRODUCT SPECIFICATIONS |
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
| For 4” x 48” product: | ||||
| 152 mm x 152 mm x 267 mm (6” x 6” x 10 1/2”) | ||||
| Inserts: | Each box contains one product insert. | |||
| Labeling: | Each box and case shall be labeled with a product code and size and lot/expiry date as defined on the Boxing and Casing Operating Specification. | |||
| One label is affixed to the bottom end of each box. |
| C. | OUTER PACKAGING (CASE) |
| Dimensions: | For 4” x 4” product: | |||
| 165 mm x 216 mm x 559 mm (6 1/2” x 8 1/2” x 22”) | ||||
| For 4” x 8” product: | ||||
| 165 mm x 318 mm x 559 mm (6 1/2” x 12 1/2” x 22”) | ||||
| For 8” x 16” product: | ||||
| 267 mm x 318 mm x 559 mm (10 1/2” x 12 1/2” x 22”) | ||||
| For 16” x 16” product: | ||||
| 267 mm x 318 mm x 559 mm (10 1/2” x 12 1/2” x 22”) | ||||
| For 4” x 48” product: | ||||
| 165 mm x 318 mm x 559 mm (6 1/2” x 12 1/2” x 22”) | ||||
| Size tolerance ± 6.35 mm (± 0.25”) | ||||
| Labeling: | Each box and case shall be labeled with a case label, product code and size and lot/expiry date as defined on the Boxing and Casing Operating Specification. The labels are affixed to each end of the case. |
| 1.5 | STERILIZATION |
1.5.1 Sterilization Method:
|
Gamma irradiation | |
1.5.2 Sterilization Verification:
|
Validation testing to achieve a XXX of 10-6. | |
1.5.3 Sterilization Special Concerns:
|
Gamma irradiation should not exceed 50 kGy for a single dose or 75 kGy for a total dose. |

|
PRODUCT SPECIFICATIONS |
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
| 2. | FUNCTIONAL ASPECTS OF DESIGN |
| 2.1. | PHYSICAL CHARACTERISTICS |
| 2.1.1. | Construction: | ||
| Three-ply dressing consisting of a piece of rayon/polyester gauze laminated between two pieces of silver coated high-density polyethylene mesh. |
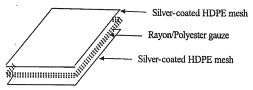
| 2.1.2. | Size: | ||
| The size, as per WB-10-02-07, is acceptable if the sample falls within the dimensions below. |
| Package Size (on label) | Dressing Width | Dressing Length | ||
| Width x Length | Dimensions | Dimensions | ||
4”x 4”
|
9.7 cm - 11.4 cm | 9.7 cm - 11.4 cm | ||
(10 cm x 10 cm) |
||||
4”x 8”
|
9.7 cm - 11.4 cm | 19.8 cm - 21.6 cm | ||
(10 cm x 20 cm) |
||||
8”x l6”
|
19.8 cm - 21.6 cm | 39.6 cm - 41.9 cm | ||
(20 cm x 40 cm) |
||||
16”x l6”
|
39.6 cm - 41.9 cm | 39.6 cm - 41.9 cm | ||
(40 cm x 40 cm) |
||||
4”x 48”
|
9.7 cm - 11.4 cm | 119.4 cm - 124.5 cm | ||
(10 cm x 120 cm) |
| 2.1.3. | Appearance |
| 2.1.3.1. | The appearance, as per WB-10-02-08, of the sample is acceptable if the following criteria are met: |
| a) | bluish side facing out on one side (top) and in on the other side (bottom) of the laminated dressing; |

|
PRODUCT SPECIFICATIONS | |||
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
| • | Has an absorbent capacity of at least 7.0 g of water/g of dressing according to procedure WB-10-03-11; and | ||
| • | Does not lose silver when subjected to an adhesion test, procedure WB-10-03-08 and an abrasion test, procedure WB-10-03-09. |
| • | For external use only | ||
| • | Should not be used on patients with a known sensitivity to silver | ||
| • | May cause transient discoloration | ||
| • | Not compatible with oil-based products, such as petrolatum | ||
| • | Not compatible with MRI (Magnetic Resonance Imaging) procedures | ||
| • | Should not come in contact with electrodes or conductive gels during electronic measurements | ||
| • | Avoid exposure to temperatures above 120°F (50°C) | ||
| • | Do not use if product color is not uniform | ||
| • | Sterile water should be used to moisten the dressing | ||
| • | Do not use if package is damaged. | ||
| • | Single use only. Do not re-sterilize |
| • | Remove the dressing from the package. | ||
| • | Moisten the dressing with sterile water (do not use saline) and cut to shape if necessary. | ||
| • | Apply the dressing to the wound surface and cover with an appropriate secondary dressing that will maintain a moist environment. | ||
| • | Keep the dressing moist, but not so wet that tissue maceration occurs. | ||
| • | Examine the dressing to ensure a moist environment is being maintained | ||
| • | Change the dressing depending on the amount of exudate and the condition of the wound. | ||
| • | If the dressing dries and adheres to the wound, moisten or soak the dressing prior to removal. | ||
| • | Avoid forceful removal and disruption of the healing wound. |
| • | The shelf life will be 30 month. | ||
| • | Maximum exposure temperature: 50°C. |
Page 9 of 17

|
PRODUCT SPECIFICATIONS | |||
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
| 3.1 | PROPOSED KEY PROCESSES (IDENTIFICATION AND DESCRIPTION OF CONTROLS) | |
| The coating of silver onto HDPE net will be carried out using a physical vapour deposition process in the Trial Manufacturing Roll Coater (TMRC). | ||
| Two layers of coated HDPE mesh will be laminated to the rayon/polyester nonwoven gauze and slit to the appropriate width using the Trial Manufacturing Ultrasonic Laminating and Slitting Machine. | ||
| The packaging of the burn dressing will be carried out using the Trial Manufacturing Packaging Machine. Each dressing will be cut to the appropriate size and packaged in a Tyvek pouch. | ||
| An approved contractor will conduct sterilization of pouches. Packing of pouches into boxes and cases will be carried out after sterilization. | ||
| 3.2 | ENVIRONMENTAL REQUIREMENTS FOR MANUFACTURE | |
| Lamination and pouching will be performed in a controlled environment room. | ||
| 3.3 | APPLICABLE QUALITY CONTROL STANDARDS | |
| The product will be manufactured in accordance with the quality practices specified in the following documents: |
| o | EN 46001:1996, Quality Systems — Medical Devices — Particular Requirements for the Application of EN29001 | ||
| o | ISO 13485:1996, Quality Systems — Medical Devices — Particular Requirements for the Application of ISO 9001 | ||
| o | Quality System Regulations specified in the Current Good Manufacturing Practices (CGMP) for medical devices; 21 CFR part 820 |
| 3.4 | PROPOSED QUALITY CONTROL TESTING / TEST REQUIREMENTS |
| 3.4.1 | Raw Materials Testing: | ||
| Raw materials should be receiving and tested as per existing QSI procedures and specifications. | |||
| 3.4.2 | Finished Device Testing: | ||
| The finished product must have the following performance characteristics: |
Page 10 of 17

|
PRODUCT SPECIFICATIONS | |||
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
| Characteristic | Procedure | Specification | ||||
| Size | WB-10-02-07 | As section 2.1.2 (but not including thickness). | ||||
| Appearance | WB-10-02-08 | As section 2.1.3 | ||||
| Colour | WB-10-02-31 | As per colour standard | ||||
| Total Silver | WB-10-02-14 | 0.84-1.34 mg/cm2 | ||||
| NH4OH soluble silver | WB-10-02-14 | ³42% of the Total Silver at the time of release | ||||
| NH4OH soluble silver | WB-10-02-14 | ³35% of the Total Silver when tested 30 months after manufacture | ||||
Pouch label appearance
|
WB-10-02-03 | a) | the label is centered on the package; | |||
| b) | except for the internal part number for the packaging material, printing must not carry over into the seam; | |||||
| c) | the lot and expiry date stamps are straight and in the bottom left corner; | |||||
| d) | the printing is readable; and | |||||
| e) | the label matches the dressing in the package. | |||||
Pouch seal size & position
|
WB-10-02-04 | a) | the seal is at least 7 mm wide, within 7 mm from the side edge and within 10 mm from the bottom edge; and | |||
| b) | the Chevron seal is at the top of the label. | |||||
| Pouch seal strength | WB-10-02-05 | burst test pressure of at least 1.0 psig. | ||||
| Pouch seal integrity | WB-10-02-02 WB-10-02-06 |
there shall be a continuous seal with no bubbles, blisters or other inconsistencies | ||||
Page 11 of 17

|
PRODUCT SPECIFICATIONS | |||
| Design Project: | Acticoat Burn Dressing | |||
| Design Version: | 2.0 | |||
| Design History File: | 11032 / 1600 |
Page 12 of 17
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat Burn Dressing | ||||
| Design Version: | 2.0 | ||||
| Design History File: | 11032 / 1600 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat Burn Dressing | ||||
| Design Version: | 2.0 | ||||
| Design History File: | 11032 / 1600 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat Burn Dressing | ||||
| Design Version: | 2.0 | ||||
| Design History File: | 11032 / 1600 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat Burn Dressing | ||||
| Design Version: | 2.0 | ||||
| Design History File: | 11032 / 1600 | ||||

| to a Supply Agreement made effective as of May 8, 2001 among | ||
| Westaim Biomedical Corp, Xxxxx & Nephew Inc, Xxxxx & | ||
| Nephew, Inc. and T. I. Xxxxx & Nephew Limited |
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| 1.0 | SUMMARY |
| 1.1 | THEORY OF OPERATION OF THE PROPOSED DESIGN |
| • | To provide seven days of usable antimicrobial performance, more silver and/or more absorbency will be required than that in the Acticoat Burn Dressing. The increased silver will be provided by the addition of a third layer of silver-coated Delnet. The additional absorbency will be provided by the addition of a second layer of Sontara. | ||
| • | The dressing will provide antimicrobial activity through a sustained release of silver, which will minimize bacterial penetration to the wound and within the dressing. | ||
| • | To maintain a moist wound environment an appropriate secondary dressing may be necessary to limit moisture evaporation. |
| 1.2 | DRAWINGS/SCHEMATICS (REFERENCE APPLICABLE ATTACHMENTS) |
| 1.3 | MATERIALS OF CONSTRUCTION |
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| 1.3.1 | No new materials of construction will be required for the Acticoat 7 Dressing. The dressing will be constructed of two layers of Sontara Style 8411 (Part #A2000) and three layers of silver coated Delnet (Part #B0010) | ||
| 1.3.2 | Vendor/Supplier, Vendor P/N All material should be purchased and received according to approved specifications and procedures listed in QSI. |
||
| 1.3.3 | Rare or Single-Source Materials None |
||
| 1.3.4 | Special Vendor Contract Requirement No additional vendor contracts are required for this product. |
| 1.4 | PACKAGING |
| 1.4.1 | PACKAGING CONFIGURATIONS |
| 1.4.2 | PACKAGING MATERIALS |
| Part number: | 4” x 5” | Front: A3070 | Back: A3000 | |||||
| 6” x 6” | Front: A3071 | Back: A3006 | ||||||
| Composition: | Front: | Tyvek 1073B (Spunbonded polyolefin) coated with SBP 2000 and printed. | ||||||
| Back: | Tyvek 1073B (Spunbonded polyolefin) uncoated and imprinted. | |||||||
| Basis Weight: | 2.57 ± 0.21 oz./sq. yd. (8.71 ± 0.71 mg/cm2) | |||||||
| Roll Widths: | 6” ± 0.25” or 8” ± 0.25” | |||||||
| Appearance: | Clean, white, and no wrinkles or tears. | |||||||
| Printing: | The images shown in Figures 1 or 2 are printed on the uncoated side of the Tyvek. The printed side should be on the outside of the roll with the top of the label facing the leader end of the roll. | |||||||
| Colours: The Pantone colours used for printing are as follows: | ||||||||
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| Yellow | 123 | |||||||||
| Blue | 286 | |||||||||
| Grey | Cool Grey 6 | |||||||||
| Black | ||||||||||
| Certification: | Technical specifications must be certified for each lot of Tyvek packaging. Supplier quality documentation that includes composition, product type, part identification (lot number), and quantity shipped must accompany each shipment. | |||||||||
| B. OUTER PACKAGING (BOX) | ||||||||||
| Part number: | 4” x 5”: A4070 6” x 6”: A4071 | |||||||||
| Composition: | Cardboard, solid bleached sulphite (0.020 SBS) with a 0.5 mil poly coated backside. | |||||||||
| Printing: | Printed with images shown in Figure 3 or 4; | |||||||||
| Colours: | The Pantone colours used for printing are as follows: | |||||||||
| Yellow | 123 | |||||||||
| Blue | 286 | |||||||||
| Grey | Cool Grey 6 | |||||||||
| Black | ||||||||||
| Appearance: | White, no wrinkles, straight folds, side glued with tuck ends. | |||||||||
| C. OUTER PACKAGING (CASE) | ||||||||||
| Part number: | 4” x 5”: A4101 6” x 6”: A4102 | |||||||||
| Composition: | Corrugated case Kraft 175B, silver, outside regular adhesive. | |||||||||
| Printing: | None; | |||||||||
| Appearance: | White exterior, no wrinkles, straight folds, side glued with tuck ends. | |||||||||
| Note : These are the same imprinted cases used for the Acticoat Burn Dressing. | ||||||||||
| D. PRODUCT INSERT | ||||||||||
| Part number: | A5006 | |||||||||
| Composition: | Hammermill Bond/wr 24 lb., white paper | |||||||||
| Dimensions: | 8.5” x 11” (21.6 cm x 28 cm) folded into 3 sections, with the English side facing out, to make 8.5” x 3.67” | |||||||||
| Printing: | The label is printed with in 6 languages as shown in Figure 5. | |||||||||
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| Colours: The Pantone colours used for printing are as follows: | ||||||
| Yellow | 123 | |||||
| Blue | 286 | |||||
| Grey | 38% Screen of Black | |||||
| Black | ||||||
| Appearance: | Clean, white, and no wrinkles or tears. | |||||
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| 1.4.3 | PACKAGING SPECIFICATIONS |
| A. PRIMARY PACKAGING (POUCH) | ||||||
| Contents: | Peelable pouch with chevron seal, each containing one dressing. | |||||
| Dimensions: | For 4” x 5” product: | 153 mm x 216 mm (6” x 81/2”) | ||||
| For 6” x 6” product: | 203 mm x 260 mm (8” x 10”) | |||||
| Size tolerance ± 6.35 mm (± 0.25”) | ||||||
| Label: | The label appearance is acceptable as per WB-10-02-03 if the following criteria are met: | |||||
| a) | the label is centered on the package; | |||||
| b) | the lot and expiry date stamps are straight and in the bottom left corner; | |||||
| c) | the printing is readable; and | |||||
| d) | the label matches the dressing in the package. | |||||
| Seal: | The seal size and position, as per WB-10-02-04, is acceptable if the following criteria are met: | |||||
| a) | the seal is at least 7 mm wide, within 7 mm from the side edge and within 10 mm from the bottom edge; and | |||||
| b) | the Chevron seal is at the top of the label. | |||||
| Seal Integrity: | A continuous seal with no bubbles, blisters or other inconsistencies as per WB-10-02-06 and WB-10-02-02. | |||||
| If channels are observed during the dye penetration test, a minimum seal width of 7 mm must be maintained to accept the results. | ||||||
| Seal Strength: | All package pouches will have a burst test pressure of at least 1.0 psig as per WB-10-02-05. | |||||
| B. OUTER PACKAGING (BOX) | ||||||
| Box style: | Notched reverse tuck end (French) carton. | |||||
Contents:
|
Each | box contains 5 dressings. | ||||
| Pouches shall be oriented with the label facing up towards the opening of the box and with the label oriented in the same direction as the box label. | ||||||
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| Dimensions: | For 4” x 5” product: | |||||
| 155 mm x 219 mm x 19 mm (6 1/8” x 8 5/8” x 0.75”) | ||||||
| For 6” x 6” product: | ||||||
| 206 mm x 257 mm x 19 mm (8 1/8” x 10 1/8” x 0.75”) | ||||||
| Inserts: | Each box contains one product insert. | |||||
| Labeling: | Each box shall be labeled with a product code and size and lot/expiry date as defined on the Boxing and Casing Operating Specification. | |||||
| One label is affixed to the bottom end of each box. | ||||||
| C. OUTER PACKAGING (CASE) | ||||||
| Dimensions: | For 4” x 5” product: | 318 mm x 165 mm x 559 mm | ||||
| (121/2” x 61/2” x 22”) | ||||||
| For 6” x 6” product: | 318 mm x 267 mm x 559 mm | |||||
| (121/2” x l01/2“x 22”) | ||||||
| Size tolerance ± 6.35 mm (± 0.25”) | ||||||
| 1.5 STERILIZATION | ||||
1.5.1
|
Sterilization Method: | Gamma irradiation | ||
1.5.2
|
Sterilization Verification: | Validation testing to achieve a XXX of 10-6. | ||
1.5.3
|
Sterilization Special Concerns: | Gamma irradiation should not exceed 50 kGy for a single dose or 75 kGy for a total dose. | ||
| 2.1 | PHYSICAL CHARACTERISTICS |
| 2.1.1 | Construction: |
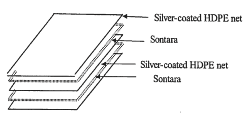
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| ¬ Silver-coated HDPE net |
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| 2.1.2 | Size: |
| Package Size (on label) | Dressing | Dressing | ||
| Width x Length | Width Dimensions | Length Dimensions | ||
4”
x 5”
|
9.7 cm— 11.4 cm | 12.2 cm —13.9 cm | ||
(10 cm x 12 cm) |
||||
6”
x 6”
|
14.7cm— 16.4cm | 14.7 cm— 16.4 cm | ||
(15 cm x 15 cm) |
| 2.1.3 | Appearance |
| 2.1.3.1 | The appearance, as per WB-10-02-08, of the sample is acceptable if the following criteria are met: |
| a) | bluish side facing out on one side (top) and in on the other side (bottom) of the laminated dressing; | ||
| b) | top side demonstrates a uniform color (if the uniformity of the blue side is in question, it must show the equivalent distance between 2 CIE data points on the ellipse is less than or equal to 0.09, as per WB-10-02-15). | ||
| c) | both sides show no strong stripes, color fading, patches or other discoloration that do not meet the specifications defined in 2.1.4. | ||
| d) | uniform pattern of attachment points with no more than three adjacent attachment points missing, and all three layers securely attached; | ||
| e) | clean-cut edges; | ||
| f) | no melted or burnt patches, rips or tears; and | ||
| g) | pitting sizes of 1/16” or less; or pitting sizes between 1/16” and 3/16” if the number of occurrences is £ 3. |
| 2.1.3.2 | Due to the subjective nature of assessing appearance conformity, the following applies: |
| a) | verify conformance to the stated specification; | ||
| b) | compare the sample to work standards when possible; | ||
| c) | if the observations are questionable the Quality Systems Manager will assess conformance to the specification; | ||
| d) | if the product does not meet predefined requirements, reject the sample; and | ||
| e) | if it is unclear whether the product meets the requirements, reject the sample and convene an MRB as per WB-13-01. |
| 2.1.4 | Colour: Due to the difficulty of measuring the product colour by the Minolta instrument, the criteria for acceptability of colour will be determined by reference to colour standards as per WB-10-02-31. The product colour must meet the requirements in the Quality Assurance Colour Standard, Revision 1, August 16, 2000. |
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| 2.1.5 | Lamination Strength |
| 2.1.5.1 | The lamination of the sample, as per WB-10-02-10, is acceptable if each layer is securely joined. |
| 2.2 | KNOWN LIMITATIONS (CAUTIONS) |
| • | For external use only; | ||
| • | Should not be used on patients with a known sensitivity to silver; | ||
| • | May cause transient discoloration; | ||
| • | Not compatible with oil-based products, such as petrolatum; | ||
| • | Not compatible with MRI (Magnetic Resonance Imaging) procedures; | ||
| • | Should not come in contact with electrodes or conductive gels during electronic measurements; | ||
| • | Avoid exposure to temperatures above 120°F (50°C); and | ||
| • | Do not use if the product colour is not uniform. | ||
| • | Do not use if package is damaged. | ||
| • | Single use only. Do not re-sterilize. |
| 2.3 | PARAMETERS OF OPERATION |
| • | Achieves at least a 4.0 log reduction of Pseudomonas aeruginosa in 30 minutes according to procedure WB-10-03-03; | ||
| • | Maintains antimicrobial activity by demonstrating 12 days plate to plate CZOI efficacy against Pseudomonas aeruginosa and Staphylococcus aureus using procedure WB-10- 03-04; | ||
| • | Has an absorbent capacity of at least 7.8 g of water/g of dressing according to procedure WB-10-03-11 | ||
| • | Does not lose silver when subjected to an adhesion test, procedure WB-10-03-08 and an abrasion test, procedure WB-10-03-09; |
| 2.4 | EQUIPMENT/MATERIALS REQUIRED FOR USE OF THE DEVICE |
| • | Scissors; | ||
| • | Appropriate secondary dressing; | ||
| • | Adhesive tape or other attachment products; and | ||
| • | Water or aqueous gels. |
| 2.5 | DIRECTIONS FOR USE |
| • | Remove Acticoat™ 7 Day Antimicrobial Dressing from the package; |
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| • | Moisten the dressing with sterile water (do not use saline) and cut to shape if necessary; | ||
| • | Apply the dressing to the wound so that the blue side faces the wound and secure with an appropriate secondary dressing that will maintain a moist environment; | ||
| • | Keep the dressing moist, but not so wet that tissue maceration occurs; | ||
| • | Change the dressing depending on the amount of exudate and the condition of the wound; | ||
| • | If the dressing dries and adheres to the wound, moisten or soak the dressing prior to removal; | ||
| • | Avoid forceful removal and disruption of the healing wound. |
| 2.6 | HANDLING AND STORAGE REQUIREMENTS |
| • | The shelf life will be 30 months. | ||
| • | Maximum exposure temperature: 50°C. |
| 3.1 | PROPOSED KEY PROCESSES (IDENTIFICATION AND DESCRIPTION OF CONTROLS) |
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| 3.2 | ENVIRONMENTAL REQUIREMENTS FOR MANUFACTURE |
| 3.3 | APPLICABLE QUALITY CONTROL STANDARDS |
| • | EN 46001:1996 Quality Systems — Medical Devices — Particular Requirements for the Application of EN29001 | ||
| • | ISO 13485:1996, Quality Systems — Medical Devices — Particular Requirements for the Application of ISO 9001 | ||
| • | Quality System Regulations specified in the Current Good Manufacturing Practices (CGMP) for medical devices; 21 CFR part 820 |
| 3.4 | PROPOSED QUALITY CONTROL TESTING / TEST REQUIREMENTS |
| 3.4.1 | Raw Materials Testing: |
| 3.4.2 | Finished Device Testing: |
| Characteristic | Procedure | Specification | ||
Size
|
WB-10-02-07 | As section 2. 1. 2 | ||
Appearance
|
WB-10-02-08 | As section 2. 1. 3 | ||
Colour
|
WB-10-02-31 | As per colour standard | ||
Total Silver
|
WB- 10-02-14 | 1.26-2.01 mg/cm2 | ||
NH4OH soluble silver
|
WB-10-02-14 | ³44% of the Total Silver at the time of release | ||
NH4OH soluble silver
|
WB-10-02-14 | ³35% of the Total Silver when tested 30 months after manufacture | ||
Pouch label appearance
|
WB-10-02-03 | As per section 1.4.3A | ||
Pouch seal size & position
|
WB-10-02-04 | As per section 1.4.3A | ||
Pouch seal strength
|
WB-10-02-05 | Burst test pressure of at least 1.0 psig |
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
Pouch seal integrity
|
WB-10-02-02 WB-10-02-06 |
There shall be a continuous seal with no bubbles, blisters or other inconsistencies |
| 3.5 | EQUIPMENT TO BE VALIDATED: | |
| NONE |
| 3.6 | PROCESSES TO BE VALIDATED: | |
| Lamination, slitting, packaging, sterilization |
| 4.0 | SHELF LIFE |
| 5.0 | APPROVAL |
| Department | Name/Signature | Title | Date | |||
Project Leader |
||||||
Marketing |
||||||
Science and
Technology |
||||||
Manufacturing |
||||||
Quality Assurance |
||||||
Regulatory Affairs |
 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||

 |
PRODUCT SPECIFICATIONS |
||||
| Design Project: | Acticoat 7 Dressing | ||||
| DHF#: | 1999 | ||||
| Design Version Number: | 2.1 | ||||
| to a Supply Agreement made effective as of May 8, 2001 among Westaim Biomedical Corp., Xxxxx & Nephew Inc., Xxxxx & Nephew, Inc. and X.X. Xxxxx & Nephew Limited |
| 1. | Roll Coater: |
| 2. | Laminating Machine: |
| 3. | Packaging Machine: |
Model No: CP-H12-P1S-TS
S/N 121
Dual Position Unwind Module
S/N 9003.826
