RESEARCH, LICENSE, AND COLLABORATION AGREEMENT between DYADIC INTERNATIONAL INC. and JANSSEN BIOTECH, INC. Dated as of [●]
Exhibit 10.1
|
Confidential |
Execution Version
Confidential
Portions of this Exhibit have been redacted because they are both (i) not material and (ii) would be competitively harmful if publicly disclosed. Information that was omitted has been noted in this document with a placeholder identified by the xxxx "[***]”.
RESEARCH, LICENSE, AND COLLABORATION AGREEMENT
between
and
XXXXXXX BIOTECH, INC.
Dated as of [●]
TABLE OF CONTENTS
|
ARTICLE 1 DEFINITIONS |
1 |
|
|
ARTICLE 2 RESEARCH PLAN ACTIVITIES |
16 |
|
|
2.1 |
Research Plan and Budget; Amendments |
16 |
|
2.2 |
Research Activities |
17 |
|
2.3 |
Compliance |
17 |
|
2.4 |
Reporting |
17 |
|
2.5 |
Records |
17 |
|
2.6 |
Research Plan Costs and Expenses |
18 |
|
2.7 |
Research Plan; Materials |
18 |
|
2.8 |
Disclosure of New Technology And Patent Rights |
19 |
|
2.9 |
Personnel Obligations |
19 |
|
2.10 |
Audit Rights |
19 |
|
2.11 |
Inspections by Regulatory Authorities |
20 |
|
ARTICLE 3 GOVERNANCE |
20 |
|
|
3.1 |
Joint Steering Committee |
20 |
|
ARTICLE 4 TARGET RESERVATION; OPTIONS AND OPTION EXERCISE |
24 |
|
|
4.1 |
Reservation of Targets |
24 |
|
4.2 |
Xxxxxxx Options |
26 |
|
4.3 |
Effects of Failure to Exercise Research License Option |
27 |
|
ARTICLE 5 LICENSE GRANTS |
27 |
|
|
5.1 |
Research Plan Licenses. |
27 |
|
5.2 |
Xxxxxxx Research License |
28 |
|
5.3 |
Xxxxxxx Commercial Licenses |
28 |
|
5.4 |
Xxxxxxx Exclusive Target License |
28 |
|
5.5 |
Unblocking Licenses |
29 |
|
5.6 |
Sublicenses |
29 |
|
5.7 |
Use of Affiliates and Contractors and Subcontractors |
30 |
|
5.8 |
Xxxxxxx Covenant |
30 |
|
5.9 |
Know-How And Technology Transfer |
30 |
|
5.10 |
In‑License Agreements |
31 |
|
ARTICLE 6 XXXXXXX PROGRAMS |
32 |
|
|
6.1 |
Development under Research License |
32 |
|
6.2 |
Commercial Programs |
35 |
|
6.3 |
Xxxxxxx Commercialization |
35 |
|
6.4 |
Xxxxxxx Diligence. |
36 |
|
6.5 |
Compliance with Applicable Law |
36 |
|
ARTICLE 7 PAYMENTS |
36 |
|
|
7.1 |
Initial Target Reservation Fee |
36 |
|
7.2 |
Research Plan Costs and Expenses |
36 |
|
7.3 |
Option Exercise Fees |
37 |
|
7.4 |
Development and Commercial Milestones by Xxxxxxx |
38 |
|
7.5 |
Production-Based Milestones |
38 |
|
7.6 |
Third Party IP |
40 |
|
7.7 |
Mode of Payment; Offsets |
41 |
|
7.8 |
Income Tax Withholding |
41 |
|
7.9 |
Indirect Taxes |
42 |
|
7.10 |
Records; Audits |
42 |
|
7.11 |
No Other Compensation |
43 |
|
7.12 |
No Limitation |
44 |
|
7.13 |
Invoicing and Payments |
44 |
|
7.14 |
Paying Agent |
44 |
|
ARTICLE 8 INTELLECTUAL PROPERTY RIGHTS |
44 |
|
|
8.1 |
Ownership of Intellectual Property Rights |
44 |
|
8.2 |
Maintenance and Prosecution of Patent Rights |
45 |
|
8.3 |
Enforcement of Patents |
46 |
|
8.4 |
Infringement Claims by Third Parties |
48 |
|
8.5 |
Invalidity, Unpatentability or Unenforceability Defenses or Actions |
49 |
|
8.6 |
Third Party Licenses and Patents |
50 |
|
8.7 |
Product Trademarks |
50 |
|
8.8 |
International Nonproprietary Name |
50 |
|
8.9 |
Inventor’s Remuneration |
51 |
|
8.10 |
Xxxxxxx Patent Rights |
51 |
|
8.11 |
Common Interest |
51 |
|
ARTICLE 9 Confidentiality AND Xxx-Xxxxxxxxxx |
00 |
|
|
9.1 |
Confidentiality Obligations |
51 |
|
9.2 |
Residual Knowledge |
51 |
|
9.3 |
Exceptions |
52 |
|
9.4 |
Permitted Disclosures |
52 |
|
9.5 |
Use of Name |
54 |
|
9.6 |
Public Announcements |
54 |
|
9.7 |
Publications |
54 |
|
9.8 |
Return of Confidential Information |
55 |
|
9.9 |
Survival |
55 |
|
ARTICLE 10 REPRESENTATIONS AND Warranties |
55 |
|
|
10.1 |
Mutual Representations and Warranties |
55 |
|
10.2 |
Additional Representations, Warranties of Dyadic |
56 |
|
10.3 |
Covenants |
58 |
|
10.4 |
DISCLAIMER OF WARRANTIES |
60 |
|
ARTICLE 11 Indemnity |
60 |
|
|
11.1 |
Indemnification of Dyadic |
60 |
|
11.2 |
Indemnification of Xxxxxxx |
61 |
|
11.3 |
Notice of Claim |
61 |
|
11.4 |
Control of Defense |
61 |
|
11.5 |
Special, Indirect, and Other Losses |
63 |
|
11.6 |
Insurance |
63 |
|
ARTICLE 12 Term and Termination |
63 |
|
|
12.1 |
Term |
63 |
|
12.2 |
Termination for Material Breach |
63 |
|
12.3 |
Termination Right by Xxxxxxx For Convenience |
63 |
|
12.4 |
Termination for Insolvency |
64 |
|
12.5 |
Rights in Bankruptcy |
64 |
|
12.6 |
Modification in Lieu of Termination |
65 |
|
12.7 |
Effects of Termination |
65 |
|
12.8 |
Remedies |
66 |
|
12.9 |
Effects of Expiration |
66 |
|
12.10 |
Accrued Rights; Surviving Obligations |
66 |
|
ARTICLE 13 DISPUTE RESOLUTION |
67 |
|
|
13.1 |
Resolution of Disputes |
67 |
|
13.2 |
Mediation |
67 |
|
13.3 |
Arbitration |
68 |
|
ARTICLE 14 Miscellaneous |
69 |
|
|
14.1 |
Force Majeure |
69 |
|
14.2 |
Export Control |
69 |
|
14.3 |
Assignment |
69 |
|
14.4 |
Severability |
70 |
|
14.5 |
Governing Law; Service |
70 |
|
14.6 |
Notices |
70 |
|
14.7 |
Entire Agreement; Amendments |
72 |
|
14.8 |
English Language |
72 |
|
14.9 |
Equitable Relief |
72 |
|
14.10 |
Waiver and Non-Exclusion of Remedies |
72 |
|
14.11 |
No Benefit to Third Parties |
72 |
|
14.12 |
Further Assurance |
72 |
|
14.13 |
Performance by Affiliates |
72 |
|
14.14 |
Relationship of the Parties |
73 |
|
14.15 |
Counterparts; Facsimile Execution |
73 |
|
14.16 |
References |
73 |
|
14.17 |
Schedules |
73 |
|
14.18 |
Construction |
73 |
SCHEDULES
|
Schedule 1.40 |
Danisco License Agreement |
|
Schedule 1.51 |
Dyadic Assigned C1Rights |
|
Schedule 1.102: |
Initial Xxxxxxx Proteins |
|
Schedule 1.103: |
Initial Reserved Targets |
|
Schedule 1.119: |
Xxxxxxx & Xxxxxxx Universal Calendar |
|
Schedule 2.1.1: |
Initial Development Plan |
|
Schedule 9.6: |
Press Release |
|
Schedule 10.2: |
Dyadic Disclosure Schedule |
|
Schedule 10.2.1: |
Existing Patents |
|
Schedule 10.2.4 |
Existing In-Licenses |
|
Schedule 5.7: |
Approved Subcontractors |
Research, License, and Collaboration Agreement
This Research, License, and Collaboration Agreement (the “Agreement”) is made and entered into as of [●] (the “Effective Date”) by and between Dyadic International Inc., (“Dyadic”), and Xxxxxxx Biotech, Inc., a Pennsylvania corporation (“Xxxxxxx”). Dyadic and Xxxxxxx are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
WHEREAS, Dyadic is a global biotechnology company with specialized expertise in developing microbial protein production technologies, including filamentous fungi, and Dyadic Controls certain intellectual property rights with respect to the C1 Platform in the Territory; and
WHEREAS, Xxxxxxx desires to collaborate with Dyadic to research and evaluate the use of the C1 Platform for the production of pharmaceutical proteins; and
WHEREAS, Dyadic wishes to grant, and Xxxxxxx wishes to take, a license to use the C1 Platform to conduct certain research activities, including the expression of certain Xxxxxxx Proteins, and an option to take a license under such intellectual property rights to use the C1 Platform to express Xxxxxxx Proteins directed to certain Targets and to Develop, Manufacture, Commercialize and otherwise Exploit such Xxxxxxx Proteins in the Field in the Territory, in each case in accordance with the terms and conditions set forth below.
NOW, THEREFORE, in consideration of the premises and the mutual promises and conditions hereinafter set forth, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties, intending to be legally bound, do hereby agree as follows:
ARTICLE 1
DEFINITIONS
Unless otherwise specifically provided herein, the following terms shall have the following meanings:
1.1 “Accessible Titers” has the meaning set forth in Section 7.5.1 (Determination of Titers For Each Xxxxxxx C1 Protein).
1.2 “Additional Reserved Targets” has the meaning set forth in Section 4.1.2(a) (Selection of Additional Targets).
1.3 “Affiliate” means, with respect to any Entity (including a Party), any other Entity controlled by, controlling, or under common control with such Entity at the time of the determination of affiliation. For the purposes of this definition, the term “control” (including, with correlative meanings, the terms “controlled by” and “under common control with”) as used with respect to an Entity means: (a) direct or indirect ownership of more than 50% of the voting securities, capital stock or other equity interests of such Entity; or (b) possession, directly or indirectly, of the power to direct the management and policies of such Entity, whether through the ownership of voting securities, by contract or otherwise.
1.4 “Agreement” has the meaning set forth in the preamble hereto.
1.5 “Alliance Manager” has the meaning set forth in Section 3.1.7 (Alliance Manager).
1.6 “Applicable Law” means federal, state, local, national and supra-national laws, statutes, rules and regulations, including any rules, regulations, regulatory guidelines or other requirements of the Regulatory Authorities, major national securities exchanges or major securities listing organizations that may be in effect from time to time during the Term and applicable to a particular activity or country or other jurisdiction hereunder, including, as applicable, the federal anti-kickback statute (42 U.S.C. § 1320a-7b), the related safe harbor regulations, and the Limitation on Certain Physician Referrals, also referred to as the “Xxxxx Law” (42 U.S.C. § 1395nn), and Physician Payments Sunshine Act at 42 U.S.C. § 1320a-7h.
1.7 “Bankruptcy Code” has the meaning set forth in Section 12.5.1 (Applicability of 11 U.S.C. § 365(n)).
1.8 “Biosimilar Application” has the meaning set forth in Section 8.3.3 (Biosimilar Applications).
1.9 “Biosimilar Product” means, with respect to a pharmaceutical or biologic product and on a country-by-country basis, a biologic product (a) whose licensing, approval or marketing authorization, or whose application for such licensing, approval or marketing authorization, relies in whole or in part on a prior approval, licensing or marketing authorization granted to such pharmaceutical or biologic product, (b) whose licensing, approval or marketing authorization, or whose application for such licensing, approval or marketing authorization, relies in whole or in part on any data generated in support of a prior approval, licensing, or marketing authorization granted to such pharmaceutical or biologic product, or (c) is determined by the FDA (or a foreign counterparty thereof) to be biosimilar to or interchangeable with such pharmaceutical or biologic product, as set forth at 42 USC 262(k)(4) (or foreign equivalent thereof). A biological product licensed, marketed, sold, manufactured, or produced by Xxxxxxx, its Affiliates, Subcontractors, or Permitted Representatives, under the same Marketing Approval Application for the Xxxxxxx C1 Protein shall not constitute a Biosimilar Product.
1.10 “BLA” has the meaning set forth in Section 1.130 (Marketing Approval Application).
1.11 “Breach Inventions” has the meaning set forth in Section 2.7.4 (Breach Inventions).
1.12 “Breaching Party” has the meaning set forth in Section 12.2 (Material Breach).
1.13 “Business Day” means a day other than Saturday, Sunday or any other day on which banking institutions in New York, New York, USA, are authorized or required to be closed for business.
1.14 “C1” means any cell or cell line derived from or comprising Thermothelomyces heterothallica (formerly Myceliophthora thermophila and originally Chrysosporium lucknowense).
1.15 “C1 Biological Target List” has the meaning set forth in Section 4.1.2(e) (C1 Biological Target List).
1.16 “C1 Expression System” means C1 engineered for the expression of exogenous proteins or peptides.
1.17 “C1 Platform” means Dyadic’s proprietary C1 Expression System and those proprietary cell lines, expression system and associated genetic elements, protocols and tools (the “C1 Tools”) used and Controlled by Dyadic to express or manufacture exogenous therapeutic proteins and polypeptides.
1.18 “C1 Tools” has the meaning set forth in Section 1.17 (C1 Platform).
1.19 “Calendar Quarter” means a quarter based on the Xxxxxxx & Xxxxxxx Universal Calendar for that quarter; provided, however, that (a) the first Calendar Quarter of the Term shall begin on the Effective Date and extend until the end of such Calendar Quarter and (b) the last Calendar Quarter of the Term shall begin on the first day of such Calendar Quarter and end on the effective date of the expiration or termination of this Agreement.
1.20 “Calendar Year” means a year based on the Xxxxxxx & Xxxxxxx Universal Calendar for that year; provided, however, that (a) the first Calendar Year of the Term shall begin on the Effective Date and end on the last day of Calendar Year 2021; and (b) the last Calendar Year of the Term shall begin on the first day of the Calendar Year in which this Agreement expires or terminates and end on the effective date of expiration or termination of this Agreement.
1.21 “CAPA” has the meaning set forth in Section 2.10 (Audit Rights).
1.22 “CDA” means that certain Confidential Disclosure Agreement between Xxxxxxx Research & Development LLC (an affiliate of Xxxxxxx) and Dyadic [***].
1.23 “Cell Line Technology Transfer” has the meaning set forth in Section 6.1.4(a) (Research Transfer).
1.24 “Cell Line Technology Transfer Fee” has the meaning set forth in Section 7.3.2 (Cell Line Technology Transfer Fee).
1.25 “Centralized Approval Procedure” means the procedure through which a MAA filed with the EMA results in a single marketing authorization valid throughout the European Union.
1.26 “Clinical Data” means all data with respect to any Xxxxxxx C1 Protein and made, collected or otherwise generated under or in connection with Clinical Trials, including any data (including raw data), reports and results with respect thereto.
1.27 “Clinical Trial” means any study in which human subjects are dosed or treated with a pharmaceutical or biological product, whether approved or investigational, including a Xxxxx 0 Xxxxx, Xxxxx 0 Xxxxx, Xxxxx 0 Trial or post-approval clinical trial.
1.28 “CMC” means chemistry, manufacturing and controls.
1.29 “CMC Development” means Development activities related to the CMC of drug substance and drug product intended to ensure the proper identification, quality, purity and strength of the drug, including test method development and stability testing, process development, drug substance development, delivery system development, technology transfer, process validation, process scale-up, formulation development, quality assurance and quality control development.
1.30 “Commercial License Option” has the meaning set forth in Section 4.2.2(a) (Reserved Targets Option).
1.31 “Commercialization” means any activities directed to marketing, promoting, detailing, distributing, importing, exporting, using, offering to sell or selling a drug or biologic product, including activities directed to obtaining Pricing and Reimbursement Approvals, conducting pre- and post-Regulatory Approval activities, conducting medical affairs activities, and launching and promoting such drug or biologic products in each country, as applicable, but expressly excluding activities directed to Development or Manufacturing. When used as a verb, “Commercialize” means to engage in Commercialization activities.
1.32 “Commercially Reasonable Efforts” [***].
1.33 “Confidential Information” of a Party means the terms of this Agreement, Data, Know-How, and any technical, scientific, business or other information (a) disclosed orally, visually, in writing or in other form by or on behalf of such Party (or an Affiliate or representative of such Party) to the other Party (or to an Affiliate or representative of such other Party) pursuant to or in connection with this Agreement on or after the Effective Date, or (b) otherwise expressly deemed to be “Confidential Information” of such Party under another provision of this Agreement.
1.34 “Control” (including, with correlative meanings, “Controls” or “Controlled by”) means, with respect to any Know-How, Patent Right, Technology or other intellectual property right, material or Confidential Information, possession by a Party (whether by ownership or license or otherwise, other than pursuant to a license or other rights granted to such Party under this Agreement), directly or through an Affiliate of such Party, of the ability to transfer or disclose, or grant a license or sublicense under, such right, material or Confidential Information, as applicable, as provided for herein without violating the terms of any agreement or binding arrangement with any Third Party.
1.35 “Convicted Entity” has the meaning set forth in Section 10.2.13(d) (Additional Representations and Warranties of Dyadic).
1.36 “Convicted Individual” has the meaning set forth in Section 10.2.13(d) (Additional Representations and Warranties of Dyadic).
1.37 “Cover(s)” (including, with correlative meanings, “Covering” and “Covered”) means, with respect to a Patent Right and a product, invention or technology, that, in the absence of ownership of or a license under such Patent Right, the manufacture, use, sale, offer for sale or importation of such product or the practice of such invention or technology would Infringe one or more claims of such Patent Right.
1.38 “CPR Mediation Procedure” has the meaning set forth in Section 13.2.1 (CPR Mediation Procedure).
1.39 “CPR Rules” has the meaning set forth in Section 13.3.1 (CPR Rules).
1.40 “Danisco License Agreement” means Pharma License Agreement, dated December 31, 2015, entered into between Danisco US Inc. and Dyadic International Inc. A redacted copy of the Danisco License Agreement is attached hereto as Schedule 1.40 (Danisco License Agreement).
1.41 “Data” means any and all results of research, preclinical and non-clinical studies, including in vitro, in vivo, and ex vivo studies, Clinical Trials and other testing of a drug, protein or product, and any and all other data generated by or on behalf of a Party related to the Development, Manufacture or Commercialization of a drug, protein or product, including biological, chemical, pharmacological, toxicological, safety, pharmacokinetic, clinical, CMC, analytical, quality control, mechanical, software, electronic and other data, results and descriptions.
1.42 “Data Security and Privacy Laws” means all Applicable Laws relating to the privacy, processing and security of Personal Data.
1.43 “Debarred Entity” has the meaning set forth in Section 10.2.13(b) (Additional Representations and Warranties of Dyadic).
1.44 “Debarred Individual” has the meaning set forth in Section 10.2.13(a) (Additional Representations and Warranties of Dyadic).
1.45 “Default Notice” has the meaning set forth in Section 12.2 (Material Breach).
1.46 “Designee” or “Designees” means, with respect to a Party, (a) such Party’s employees, agents, independent contractors, or consultants, (b) such Party’s Affiliates, licensees, Sublicensees, or Subcontractors, (c) the employees, agents, independent contractors, or consultants of the foregoing (b), or (d) as applicable, any other persons contractually required to assign or license Know-How, Patent Rights or Technology to such Party or any Affiliate of such Party.
1.47 “Development” means all research and non-clinical and clinical drug development activities and processes, including toxicology, pharmacology, project management and other non-clinical efforts, formulation development, CMC Development, delivery system development, statistical analysis, Manufacturing development, the performance of Clinical Trials (including the Manufacturing of products for use in Clinical Trials), or other activities reasonably necessary in order to obtain and maintain Marketing Approval of a pharmaceutical or biologic product. When used as a verb, “Develop” means to engage in Development activities, but expressly excluding activities directed to Manufacturing or Commercialization. For the avoidance of doubt, the term “Development” shall include research and activities relating to the transfection, transformation or modification of C1 to express a purposefully introduced genetic component.
1.48 “Dispute” has the meaning set forth in Section 13.1 (Resolution of Disputes).
1.49 “Dollars” or “$” means United States Dollars.
1.50 “Dyadic” has the meaning set forth in the preamble hereto.
1.51 “Dyadic Assigned C1 Rights” [***].
1.52 “Dyadic Background Patent Rights” [***]
1.53 “Dyadic Background Technology” [***]
1.54 “Dyadic Indemnitees” has the meaning set forth in Section 11.1 (Indemnification of Dyadic).
1.55 “Dyadic Internal Program” [***].
1.56 “Dyadic Owned Rights” [***]
1.57 “Dyadic Prosecuted Patent Rights” has the meaning set forth in Section 8.2.1 (By Dyadic).
1.58 “Dyadic Research Activities” has the meaning set forth in Section 2.2.1 (Dyadic Research Activities).
1.59 “Dyadic Unblocking Rights” [***]
1.60 “Dyadic Target/Protein IP” has the meaning set forth in Section 1.53 (Dyadic Background Technology).
1.61 “Effective Date” means the effective date of this Agreement as set forth in the preamble hereto.
1.62 “EMA” means the European Medicines Agency and any successor agency(ies) or authority thereto in the EU having substantially the same function.
1.63 “Entity” means any corporation, general partnership, limited partnership, limited liability partnership, joint venture, estate, trust, company (including any limited liability company or joint stock company), firm or other enterprise, association, organization or entity.
1.64 “EU” means the European Union, as its membership may be constituted from time to time, and any successor thereto; except that, for purposes of this Agreement, the EU will be deemed to include France, Germany, Italy, Spain and the United Kingdom, irrespective of whether any such country is a member state of the European Union.
1.65 “Euros” or “€” means the official currency of 19 of the 27 member states of the European Union.
1.66 “Evaluation Period” has the meaning set forth in Section 5.9.1 (Initial Technology Transfer).
1.67 “Excluded Entity” has the meaning set forth in Section 10.2.13(c) (Additional Representations and Warranties of Dyadic).
1.68 “Excluded Individual” has the meaning set forth in Section 10.2.13(c) (Additional Representations and Warranties of Dyadic).
1.69 “Exclusive Target License” has the meaning set forth in Section 5.4 (Xxxxxxx Exclusive Target License).
1.70 “Exclusive Target Option” has the meaning set forth in Section 4.2.3(a) (Exclusive Target Option Grant).
1.71 “Exclusive Target Option Exercise Date” has the meaning set forth in Section 4.2.3(b) (Exercise of Exclusive Target Option).
1.72 “Exclusive Target Option Exercise Fee” has the meaning set forth in Section 7.3.4(b) (Exclusive Target Option Exercise Fee).
1.73 “Exclusive Target Option Grant Date” has the meaning set forth in Section 4.2.3(a) (Exclusive Target Option Grant).
1.74 “Exclusive Target Option Grant Fee” has the meaning set forth in Section 7.3.4(a) (Exclusive Target Option Grant Fee).
1.75 “Exclusive Target Option Period” has the meaning set forth in Section 4.2.3(b) (Exercise of Exclusive Target Option).
1.76 “Executive Officers” has the meaning set forth in Section 13.1 (Resolution of Disputes).
1.77 “Existing In-License” has the meaning set forth in Section 10.2.4 (Additional Representations and Warranties of Dyadic).
1.78 “Existing Patents” has the meaning set forth in Section 10.2.1 (Additional Representations and Warranties of Dyadic).
1.79 “Exploit” or “Exploitation” means to make, have made, import, have imported, export, have exported, use, have used, sell, have sold, offer for sale or otherwise exploit, including to research, Develop, Commercialize, register, modify, enhance, improve, Manufacture, have Manufactured, hold, keep (whether for disposal or otherwise) or otherwise dispose of.
1.80 “FDA” means the United States Food and Drug Administration and any successor agency(ies) or authority having substantially the same function.
1.81 “Field” [***].
1.82 “First Commercial Sale” means, with respect to a Xxxxxxx C1 Protein in a country, the first commercial sale in an arm’s length transaction of such Xxxxxxx C1 Protein to a Third Party in such country after Regulatory Approval and, if applicable, Pricing and Reimbursement Approval, of such Xxxxxxx C1 Protein has been granted, or such marketing and sale is otherwise permitted, by the Regulatory Authority of such country. Sales for Clinical Trial purposes, early access programs, named patient, registration samples or compassionate use shall not constitute a First Commercial Sale. In addition, sales of a Xxxxxxx C1 Protein by and between Xxxxxxx and its Affiliates shall not constitute a First Commercial Sale.
1.83 “FTE” means the equivalent of a full-time employee’s work time over a 12-month period ([***]) working directly on performing Development activities according to the Research Plan or performing additional support activities set forth in this Agreement. For clarity, no more than [***] per Calendar Year (or equivalent pro-rata portion thereof for a period less than 12 months) may be charged for a single individual contributing work factoring into any reimbursable FTE Costs hereunder, regardless of how much additional work time is contributed by such individual during such period. An individual contributing work for less than [***] per Calendar Year shall be deemed a fraction of an FTE on a pro-rata basis.
1.84 “FTE Costs” means the amount calculated by multiplying the applicable FTE Rate by the number of FTEs expended by Dyadic in the performance of the Dyadic Research Activities under the Research Plan during the applicable financial period.
1.85 “FTE Rate” means (a) with respect to Dyadic Research Activities conducted under the Research Plan, [***], or (b) with respect to additional support activities conducted by Dyadic under this Agreement, U.S. [***], in each case ((a) and (b)), per FTE per Calendar Year (pro-rated for the period beginning on the Effective Date and ending on the last day of the first Calendar Year of the Term). The Parties acknowledge that the FTE Rate covers all salary, other compensation, employee benefits and routine supplies and includes overhead for or directly allocable to an FTE. Beginning on January 1, 2023, the FTE Rate shall be adjusted annually, based on changes in the Consumer Price Index for all Urban Consumers for July of the prior year to July of the current year, as published by the U.S. Department of Labor, Bureau of Labor Statistics, available at xxxx://xxx.xxx.xxx/xxx/xxxx.xxx.
1.86 “Gatekeeper” has the meaning set forth in Section 4.1.2(d) (Gatekeeper).
1.87 “Good Clinical Practice” or “GCP” means the current standards for clinical trials of pharmaceuticals, as set forth in the ICH guidelines and applicable regulations promulgated thereunder, as amended from time to time, and such standards of good clinical practice as are required by the EU and other organizations and Governmental Authorities in countries in which a pharmaceutical product is intended to be sold to the extent such standards are not less stringent than U.S. Good Clinical Practice.
1.88 “Good Laboratory Practice” or “GLP” means the current standards for laboratory activities for pharmaceuticals, as set forth in the FDA’s Good Laboratory Practice regulations or the Good Laboratory Practice principles of the Organization for Economic Co-Operation and Development, as amended from time to time, and such standards of good laboratory practice as are required by the EU and other organizations and Governmental Authorities in countries in which a pharmaceutical product is intended to be sold, to the extent such standards are not less stringent than U.S. Good Laboratory Practice.
1.89 “Good Manufacturing Practice” or “GMP” means the part of quality assurance that ensures that pharmaceutical products are consistently produced and controlled in accordance with the quality standards appropriate to their intended use as defined in 21 C.F.R. § 210 and 211, European Directive 2003/94/EC, Eudralex 4, Annex 16, and applicable U.S., EU, Canadian and ICH guidance or equivalent laws in other jurisdictions.
1.90 “Governmental Authority” means any applicable government authority, court, tribunal, arbitrator, agency, department, legislative body, commission or other instrumentality of (a) any government of any country or territory, (b) any nation, state, province, county, city or other political subdivision thereof or (c) any supranational body.
1.91 “Gross Mass” [***].
1.92 “Improvement” means improvement, modification, refinement, correction, evolution or enhancement and any discoveries or Inventions relating thereto made by either Party, its Affiliates or Designee who obtains, directly or indirectly, access to (a) with respect to Dyadic, Xxxxxxx Background Rights or Xxxxxxx’x Materials, and (b) with respect to Xxxxxxx, Dyadic Background Technology or Dyadic’s Materials.
1.93 “In-License Agreement” means any (a) Existing In-License or (b) agreement between Dyadic or its Affiliate, on one hand, and a Third Party on the other hand under which Xxxxxxx is granted a sublicense or other right under this Agreement as provided in Section 5.10.2 (New In‑License Agreements) or Section 7.6 (Third Party IP).
1.94 “IND” means (a) any Investigational New Drug Application, as defined in the U.S. Federal Food, Drug and Cosmetics Act, filed with the FDA pursuant to Part 312 of Title 21 of the U.S. Code of Federal Regulations, including any amendments thereto; or (b) any comparable filing(s) outside the U.S. (such as a clinical trial authorization in the EU) necessary to commence Clinical Trials, including any amendments thereto.
1.95 “IND-Enabling Study” means any non‑clinical study of a drug or biologic product (a) that is intended to comply with GLP or (b) the results of which are necessary to support the filing of an IND for such drug or biologic product.
1.96 “Indemnification Claim Notice” has the meaning set forth in Section 11.3 (Notice of Claim).
1.97 “Indemnified Party” has the meaning set forth in Section 11.3 (Notice of Claim).
1.98 “Indication” means, with respect to a product, a use to which such product is intended to be put for the treatment, prevention or cure of a distinct recognized disease or condition, or of a manifestation of a recognized disease or condition, which, if approved in the U.S., would be reflected in the “Indications and Usage” section of labeling pursuant to 21 C.F.R. §201.57(c)(2) or, to the extent applicable, any comparable labeling section outside the U.S.
1.99 “Indirect Taxes” has the meaning set forth in Section 7.9 (Indirect Taxes).
1.100 “Infringe” or “Infringement” means any infringement as determined by Applicable Law, including direct infringement, contributory infringement or any inducement to infringe.
1.101 “Initial Agreement Term” [***].
1.102 “Initial Xxxxxxx Proteins” means the Xxxxxxx Proteins identified on Schedule 1.102 (Initial Xxxxxxx Proteins).
1.103 “Initial Reserved Targets” means the [***] biological Targets set forth on Schedule 1.103 (Initial Reserved Targets), which include the biological Targets to which each of the Initial Xxxxxxx Proteins is directed, [***].
1.104 “Initiation” (including, with correlative meanings, “Initiates” or “Initiated”) means, with respect to a Clinical Trial, the first dosing of the fifth subject or patient in such Clinical Trial.
1.105 “Inspected Site” has the meaning set forth in Section 2.11 (Audits by Regulatory Authorities).
1.106 “Intellectual Property” has the meaning set forth in Section 12.5.1 (Applicability of 11 U.S.C. § 365(n)).
1.107 “Invention” means any invention, whether or not patented, that is made, conceived, generated or reduced to practice, in whole or in part, by or on behalf of either Party or jointly by or on behalf of the Parties, including with its Designees, in the course or as a result of the conduct of activities under this Agreement.
1.108 “Xxxxxxx” has the meaning set forth in the preamble hereto.
1.109 “Xxxxxxx Assigned Protein Rights” [***].
1.110 “Xxxxxxx Background Rights” [***].
1.111 “Xxxxxxx C1 Protein” [***].
1.112 “Xxxxxxx Commercial License” has the meaning set forth in Section 5.3 (Xxxxxxx Commercial Licenses).
1.113 “Xxxxxxx Exclusive Target” means [***], the Target to which the monoclonal antibody specified as one of the Initial Xxxxxxx Proteins binds.
1.114 “Xxxxxxx Indemnitees” has the meaning set forth in Section 11.2 (Indemnification of Xxxxxxx).
1.115 “Xxxxxxx Owned Rights” [***].
1.116 “Xxxxxxx Protein” [***].
1.117 “Xxxxxxx Research Activities” has the meaning set forth in Section 2.2.2 (Xxxxxxx Research Activities).
1.118 “Xxxxxxx Research License” has the meaning set forth in Section 5.2 (Xxxxxxx Research License).
1.119 “Xxxxxxx & Xxxxxxx Universal Calendar” means the Xxxxxxx & Xxxxxxx universal calendar for a given year. The Xxxxxxx & Xxxxxxx Universal Calendar for 2021 and 2022 is set forth on Schedule 1.119 (Xxxxxxx & Xxxxxxx Universal Calendar) attached hereto, and the Xxxxxxx & Xxxxxxx Universal Calendar for subsequent years will be provided to Dyadic by Xxxxxxx upon Dyadic’s request in writing from time to time.
1.120 “Joint Patent Committee” or “JPC” has the meaning set forth in Section 3.1.9(c) (Joint Patent Committee).
1.121 “Joint Steering Committee” or “JSC” has the meaning set forth in Section 3.1.1 (Formation).
1.122 “Jointly Owned Rights” [***].
1.123 “Know-How” means commercial, technical, scientific and other information and know-how, including: (a) biological, chemical, pharmacological, toxicological, clinical, nonclinical, preclinical, manufacturing, safety, analytical, quality control and clinical data; (b) genetic elements, tools, reagents (e.g., plasmids, proteins, cell lines, assays and compounds); (c) compositions, machines, articles of manufacture; (d) trade secrets; (e) methods; (f) techniques, skills, instructions and formulae, designs, drawings, assembly procedures, computer programs, apparatuses, specifications, data, results; (g) processes, protocols, and practices; (h) procedures; (i) specifications; (j) sourcing information and (k) Inventions; in each case ((a)-(k)), whether patentable or not.
1.124 “Licensed Intellectual Property” [***].
1.125 “Losses” has the meaning set forth in Section 11.1 (Indemnification of Dyadic).
1.126 “MAA” has the meaning set forth in Section 1.130 (Marketing Approval Application).
1.127 “Major Market” [***].
1.128 “Manufacture” and “Manufacturing” means all activities related to the synthesis, making, having made, expressing, producing, processing, sourcing, purifying, formulating, filling, finishing, packaging, labeling, shipping and holding of a drug or biologic product or any intermediate thereof, including process development, process qualification and validation, scale-up, pre-clinical, clinical and commercial production and analytic development, product characterization, stability testing, quality assurance and quality control, but expressly excluding activities directed to Development or Commercialization.
1.129 “Marketing Approval” means approval of a Marketing Approval Application by the applicable Regulatory Authority.
1.130 “Marketing Approval Application” means: (a) a Biologics License Application submitted to the FDA pursuant to Section 351(a) of the Public Health Service Act (“BLA”) or a New Drug Application (as more fully defined in 21 CFR 314.5, et seq.) filed with the FDA, or any successor application thereto in the U.S. (“NDA”); (b) an application for authorization to market or sell a pharmaceutical or biological product submitted to a Regulatory Authority in any country or jurisdiction other than the U.S., including, with respect to the EU, a marketing authorization application filed with the EMA pursuant to the Centralized Approval Procedure or with the applicable Regulatory Authority of a country in the European Economic Area with respect to the decentralized procedure, mutual recognition or any national approval procedure (“MAA”); or (c) with respect to any product for which a BLA, NDA or MAA has been approved by the applicable Regulatory Authority, an application to supplement or amend such BLA, NDA or MAA to expand the approved label for such pharmaceutical or biological product to include use of such pharmaceutical or biological product for an additional indication.
1.131 “Materials” has the meaning set forth in Section 2.7.1 (Use of Materials).
1.132 “NDA” has the meaning set forth in Section 1.130 (Marketing Approval Application).
1.133 “Non-Breaching Party” has the meaning set forth in Section 12.2 (Material Breach).
1.134 “Occupied Target” has the meaning set forth in Section 4.1.2(c) (Occupied Targets).
1.135 “Out-of-Pocket Expenses” means amounts paid by or on account of Dyadic to Third Party vendors or contractors for supplies and materials for use, or for services provided by them, directly in the performance of Dyadic Research Activities. For clarity, Out-of-Pocket Expenses do not include payments for Dyadic’s or its Affiliates’ employee salaries, benefits, utilities, travel expenses, general office supplies, insurance, information technology or capital expenditures.
1.136 “Party” and “Parties” has the meaning set forth in the preamble hereto.
1.137 “Patent Rights” means any and all (a) patents, (b) pending patent applications, including all provisional applications, substitutions, continuations, continuations-in-part, divisions and renewals, and all patents granted thereon and registrations thereof, (c) all patents-of-addition, reissues, reexaminations and extensions, restorations, revalidations, or adjustments by existing or future extension, restoration or adjustment mechanisms, including supplementary protection certificates or the equivalent thereof, (d) inventor’s certificates, (e) other forms of government-issued right substantially similar to any of the foregoing, and (f) all U.S. and foreign counterparts of any of the foregoing.
1.138 “Permitted Representatives” means Third Parties who Manufacture pharmaceutical proteins (including intermediates that are subsequently processed into a pharmaceutical protein) on behalf of Xxxxxxx under Xxxxxxx’x “have made” rights hereunder, or any other Subcontractors or sublicensees of Xxxxxxx to whom Xxxxxxx is not prohibited from granting a sublicense or “have made,” or “have sold,” rights under the rights licensed to Xxxxxxx under ARTICLE 5 (License Grants) based on the Danisco License Agreement.
1.139 “Person” means any natural person or Entity.
1.140 “Personal Data” means (a) all information identifying, or in combination with other information, identifiable to an individual, including pseudonymized (key-coded) Clinical Data containing such information; and (b) any other information that is governed, regulated or protected by one or more Data Security and Privacy Laws.
1.141 “Phase 1 Trial” means a human clinical trial that would satisfy the requirements for a Phase 1 study as defined in 21 CFR § 312.21(a), or any foreign equivalent thereof.
1.142 “Phase 2 Trial” means a human clinical trial that would satisfy the requirements for a Phase 2 study as defined in 21 CFR § 312.21(b), or any foreign equivalent thereof.
1.143 “Phase 3 Trial” means a human clinical trial that would satisfy the requirements for a Phase 3 study as defined in 21 CFR § 312.21(c), or any foreign equivalent thereof.
1.144 “PHSA” means the United States Public Health Service Act, as amended from time to time.
1.145 “Pre-Existing Restriction” has the meaning set forth in Section 4.1.2(c) (Occupied Targets).
1.146 “Pricing and Reimbursement Approvals” means, with respect to a country or regulatory jurisdiction outside of the U.S., any pricing and reimbursement approvals that are reasonably necessary for Xxxxxxx or its Affiliate, Subcontractor, Permitted Representative to conduct a launch of a Xxxxxxx C1 Protein in such country (even if such approvals are not legally required for Xxxxxxx to conduct a launch of such Xxxxxxx C1 Protein in such country).
1.147 “Process” has the meaning set forth in Section 6.1.4(a) (Research Transfer).
1.148 “Product Infringement” has the meaning set forth in Section 8.3.1 (Enforcement of Dyadic Patents).
1.149 “Product Labeling” means, with respect to a Xxxxxxx C1 Protein in a country or other jurisdiction in the Territory, (a) the Regulatory Authority‑approved full prescribing information for such Xxxxxxx C1 Protein for such country or other jurisdiction, including any required patient information, and (b) all labels and other written, printed or graphic matter upon a container, wrapper or any package insert utilized with or for such Xxxxxxx C1 Protein in such country or other jurisdiction.
1.150 “Product Trademarks” means the Trademark(s) to be used by Xxxxxxx or its Affiliates or its or their respective Sublicensees for the Development, Commercialization or Exploitation of a particular Xxxxxxx C1 Protein in the Territory and any registrations thereof or any pending applications relating thereto in the Territory (excluding, in any event, any trademarks, service marks, names or logos that include any corporate name or logo of the Parties or their Affiliates).
1.151 “Production-Based Milestone Payments” has the meaning set forth in Section 7.5 (Production Based Milestones).
1.152 “Program” means each individual program of activities with respect to the Development of a process to produce a specific Xxxxxxx Protein directed to a Target using the C1 Platform conducted by or on behalf of either or both Parties and their Designees in accordance with this Agreement and the subsequent Manufacture or Commercialization of such Xxxxxxx Proteins by or on behalf of Xxxxxxx and its Affiliates or Permitted Representatives. Each “Program” will refer to each such set of activities for one specific Xxxxxxx Protein.
1.153 “Program Exercise Date” has the meaning set forth in Section 4.2.2(b) (Exercise of Commercial License Options).
1.154 “Program Exercise Fee” has the meaning set forth in Section 7.3.2 (Program Exercise Fees for Commercial License Grants).
1.155 “Program Payment Term” means, with respect to a Xxxxxxx C1 Protein, the period of time beginning on the First Commercial Sale of such Xxxxxxx C1 Protein in the first Major Market and ending [***] after such First Commercial Sale of such Xxxxxxx C1 Protein in such Major Market.
1.156 “Proposed In-Licensed Rights” has the meaning set forth in Section 5.10.2 (New In-License Agreements).
1.157 “Regulatory Approval” means, with respect to any product in any jurisdiction, any and all approvals (including INDs and Marketing Approvals), licenses (including import licenses), registrations and authorizations from any Regulatory Authority that are required under Applicable Law or reasonably necessary for the Development, Manufacture or Commercialization of such product in such jurisdiction for one or more uses, and all amendments and supplements thereto.
1.158 “Regulatory Authority” means any Governmental Authority, including the FDA and EMA, that has authority over the Development, Manufacture or Commercialization of pharmaceutical or biological products in a given jurisdiction in the Territory.
1.159 “Regulatory Documentation” means: (a) all applications for Regulatory Approval (including Marketing Approval Applications); (b) all Regulatory Approvals, including INDs and Marketing Approvals; (c) all supporting documents created for, referenced in, submitted to or received from an applicable Regulatory Authority relating to any of the applications or Regulatory Approvals described in clauses (a) and (b), including drug master files (or any equivalent thereof outside the U.S.), annual reports, regulatory drug lists, advertising and promotion documents shared with Regulatory Authorities, adverse event files, complaint files and Manufacturing records; and (d) all correspondence made to, made with or received from any Regulatory Authority (including written and electronic mail correspondence and minutes from meetings, discussions or conferences (whether in person or by audio conference or videoconference)).
1.160 “Reimbursable Research Costs” has the meaning set forth in Section 7.2 (Research Plan Costs and Expenses).
1.161 “Research Activities” means, as applicable, either or both of the Xxxxxxx Research Activities and the Dyadic Research Activities.
1.162 “Research Budget” has the meaning set forth in Section 2.1.2 (Research Budget).
1.163 “Research License Option” has the meaning set forth in Section 4.2.1(a) (Grant of Research License Option).
1.164 “Research License Option Exercise Date” has the meaning set forth in Section 4.2.1(a) (Grant of Research License Option).
1.165 “Research License Option Exercise Fee” has the meaning set forth in Section 7.3.1 (Research License Option Exercise Fee).
1.166 “Research Plan” has the meaning set forth in Section 2.1.1 (Research Plan).
1.167 “Research Term” means the period beginning on the Effective Date and, subject to earlier termination of this Agreement, ending on the earlier of (a) the date that is [***] following the Effective Date and (b) the Research License Option Exercise Date, as such period may be extended by agreement of the Parties.
1.168 “Reserved Targets” has the meaning set forth in Section 4.1.2(a) (Selection of Additional Targets).
1.169 “Senior Officer” means, with respect to Dyadic, its Chief Executive Officer or his/her designee, and with respect to Xxxxxxx, its Global Head Biotherapeutics Development or his/her designee.
1.170 “Subcontractor” means a Third Party contractor engaged by a Party or its Affiliates to perform certain obligations on a fee-for-service basis or exercise certain rights on behalf of and for the benefit of such Party or its Affiliates, in each case, under this Agreement (including Third Party distributors, contract research organizations, and contract manufacturing organizations).
1.171 “Sublicensee” means a Person that is granted a sublicense by Xxxxxxx under the licenses granted to Xxxxxxx under ARTICLE 5 (License Grants) as provided in Section 5.6 (Sublicenses).
1.172 “Successful Cell Line Transfer” [***].
1.173 “Target” [***].
1.174 “Target Reservation Inquiry” has the meaning set forth in Section 4.1.2(b) (Target Reservation Process).
1.175 “Target Reservation Notice” has the meaning set forth in Section 4.1.2(b) (Target Reservation Process).
1.176 “Target Selection Period” [***].
1.177 “Tax” or “Taxes” means any present or future taxes, levies, imposts, duties, charges, assessments or fees of any nature (including any interest thereon).
1.178 “Technology” means compositions, processes, machines, articles of manufacture, including genetic elements and tools. As applicable to the context, the term “Technology” will encompass C1, the C1 Platform, and the C1 Tools.
1.179 “Term” has the meaning set forth in Section 12.1 (Term).
1.180 “Terminated Territory” means each (a) each country or other jurisdiction with respect to which this Agreement is terminated by Xxxxxxx pursuant to Section 12.3 (Termination Right by Xxxxxxx For Convenience), or, (b) if this Agreement is terminated in its entirety, the entire Territory.
1.181 “Territory” means the entire world.
1.182 “Third Party” means any Person other than Dyadic, Xxxxxxx and their respective Affiliates.
1.183 “Third Party Claims” has the meaning set forth in Section 11.1 (Indemnification of Dyadic).
1.184 “Third Party IP” means Know-How or a Valid Claim of Patent Rights Controlled by a Third Party that is not licensed in a manner to Xxxxxxx such that the Development, Manufacture or Commercialization of any Xxxxxxx C1 Protein by Xxxxxxx, its Affiliates, Subcontractors or Permitted Representatives would be an act of infringement or misappropriation.
1.185 “Trademark” means any word, name, symbol, color, designation or device or any combination thereof that functions as a source identifier, including any trademark, trade dress, brand xxxx, service xxxx, trade name, brand name, logo, business symbol or domain names, whether or not registered, and any registrations thereof or any pending applications relating thereto.
1.186 “Transformed Cell Line” means C1 transformed with a gene sequence encoding a Xxxxxxx Protein as set forth in the Research Plan.
1.187 “United States” or “U.S.” means the United States of America and its territories and possessions (including the District of Columbia and Puerto Rico).
1.188 “Valid Claim” means a claim of any issued and unexpired patent which has not been held unenforceable, unpatentable, or invalid by a final non-appealable decision of a court or governmental body of competent jurisdiction.
1.189 “Working Group” has the meaning set forth in Section 3.1.9 (Working Groups).
ARTICLE 2
RESEARCH PLAN ACTIVITIES
2.1 Research Plan and Budget; Amendments.
2.1.1 Research Plan. During the Research Term, the Parties will conduct research activities in accordance with an agreed upon written research plan that will describe in reasonable detail the activities to be conducted by or on behalf of Dyadic and by or on behalf of Xxxxxxx and the deliverables thereof (as such research plan may be amended from time to time in accordance with this Agreement, the “Research Plan”). The initial Research Plan agreed to by the Parties as of the Effective Date is attached hereto as Schedule 2.1.1 (Initial Research Plan). [***].
2.1.2 Research Budget. The Research Plan will include a written budget pursuant to which Dyadic or its respective pre-approved Subcontractors will perform the Dyadic Research Activities, which budget will include a good-faith estimate of (a) the number of FTEs to be dedicated by Dyadic under the Research Plan and (b) any direct Out-of-Pocket Expenses expected to be incurred in the performance of such Dyadic Research Activities to the extent specifically identified in the Research Plan, including the costs of materials, which estimated FTEs and Out-of-Pocket Expenses will be broken down by Calendar Quarter (such budget, the “Research Budget”). All internal personnel and resources of Dyadic under each Research Budget will be expressed in terms of FTEs plus any direct Out-of-Pocket Expenses to be incurred (e.g., from the use of Subcontractors or other contract research organizations) in connection with the performance of the Dyadic Research Activities as outlined in the Research Plan and such budgeted costs will be calculated using the applicable FTE Rate. The Parties agree and acknowledge that the Research Budget will solely include such FTE Costs and Out-of-Pocket Expenses incurred by Dyadic on and after the Effective Date. The initial Research Budget agreed to by the Parties as of the Effective Date is included in the initial Research Plan attached hereto as Schedule 2.1.1 (Initial Research Plan). The JSC will review, propose modifications as appropriate to adjust for timing of performance of Dyadic Research Activities, and approve, the Research Budget each Calendar Quarter in connection with the quarterly JSC meeting.
2.1.3 Amendments to Research Plan. From time to time during the Research Term, either Party or the JSC (including in connection with its quarterly review of the Research Budget) may propose an amendment to the then-current Research Plan or Research Budget, so long as such amendment, if approved, would amend the Research Plan or Research Budget in a manner consistent with the requirements of this Agreement. The JSC shall discuss any such proposed amendment in good faith, and each Party shall ensure that its JSC representatives reasonably consider any concerns and opinions of the other Party’s JSC representatives in good faith when deciding whether to approve such proposed amendment to the Research Plan or Research Budget. Amendments to, as applicable, the Research Plan or Research Budget will become effective upon the JSC’s approval thereof. If the JSC cannot, or does not, reach consensus on such amendment, such dispute shall be subject to the dispute resolution procedures set forth in Section 3.1.5 (Dispute Resolution). Without limiting the JSC’s right to approve amendments to the Research Plan, the Party to whom a particular activity is allocated under the Research Plan will have the right, without seeking JSC approval, to make operational decisions with respect to the performance of such activity to the extent consistent with the then-current Research Plan; provided that such discretion shall not waive, modify, or otherwise decrease any diligence obligations of such Party set forth herein.
2.2 Research Activities.
2.2.1 Dyadic Research Activities. Dyadic shall (a) perform all activities allocated to Dyadic set forth in the Research Plan (the “Dyadic Research Activities”) and (b) use Commercially Reasonable Efforts to achieve the objectives, and on the applicable timelines, as set forth therein, at all times in accordance with the terms of this Agreement and the Research Plan (including the Research Budget therein).
2.2.2 Xxxxxxx Research Activities. Xxxxxxx shall (a) perform all activities allocated to Xxxxxxx set forth in the Research Plan (the “Xxxxxxx Research Activities”) and (b) use Commercially Reasonable Efforts to achieve the objectives, and on the applicable timelines, as set forth therein at Xxxxxxx’x sole cost and expense and at all times in accordance with the terms of this Agreement and the Research Plan. In connection with the Xxxxxxx Research Activities conducted under the Research Plan during the Research Term, [***].
2.3 Compliance. Each Party shall perform, and cause its Affiliates and Subcontractors to perform, its Research Activities in good scientific manner and in compliance with all Applicable Laws, including, as applicable, those relating to GLP, GCP, GMP and pharmacovigilance and safety reporting.
2.4 Reporting. [***].
2.5 Records. Each Party shall, and shall ensure that its Designees, prepare and maintain complete and accurate records in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes, and in compliance with Applicable Law, which shall properly reflect all work done, Data collected, and results achieved in the performance of its Research Activities and any Invention made in whole or in part by or on behalf of each Party in connection therewith. Such records shall be retained by each Party for at least three years after the termination or expiration of this Agreement, or for such longer period as may be required by Applicable Law. Upon either Party’s request, the other Party shall provide copies of the records it has maintained pursuant to this Section 2.5 (Records) to the requesting Party. Either Party shall have the right, during normal business hours and upon reasonable notice, to inspect and copy all records of the other Party maintained pursuant to Section 2.5 (Records). Upon Xxxxxxx’x request, Dyadic shall also promptly procure Xxxxxxx with reasonable access to relevant premises, books, records (including raw data and any correspondence with any Regulatory Authority), databases, staff and consultants of Dyadic working for Dyadic with respect to the Xxxxxxx Proteins or as required to conduct due diligence to determine whether to exercise Research License Option and shall provide to Xxxxxxx copies of all agreements that are material to the use of the C1 Platform to produce Xxxxxxx Proteins in accordance with its rights under this Agreement. Each Party shall maintain all Confidential Information of the other Party disclosed to it under this Section 2.5 (Records) in confidence in accordance with ARTICLE 9 (Confidentiality and Non-Disclosure). Xxxxxxx shall reimburse Dyadic for any out-of-pocket costs incurred for compliance with additional diligence and documentation requests from Xxxxxxx (beyond the results and Data achieved in the performance of its Research Activities and any Invention made in whole or in part by or on behalf of each Party in connection therewith) under this Section 2.5 (Records) by any Third Party and each Party shall compensate the other Party at the applicable FTE Rate for any time spent by its or its Designee’s employees above fifty hours complying with such requests. For the avoidance of doubt, nothing herein shall require Dyadic to disclose or permit access to any premises, books, records (including raw data and any correspondence with any Regulatory Authority), databases, staff and consultants that are (a) proprietary to a Third Party and that Dyadic is not permitted to disclose to Xxxxxxx or (b) unrelated to a Xxxxxxx Protein, including where such items and staff are Dyadic’s, without good cause shown in writing by Xxxxxxx. Xxxxxxx’x right to request additional diligence and documentation (beyond the results and Data achieved in the performance of its Research Activities and any Invention made in whole or in part by or on behalf of each Party in connection therewith) under this Section 2.5 (Records) shall expire at the end of the Initial Agreement Term.
2.6 Research Plan Costs and Expenses. Xxxxxxx shall reimburse Dyadic in accordance with Section 7.2 (Research Plan Costs and Expenses) for the FTE Costs and Out-of-Pocket Expenses of all Dyadic Research Activities performed by or on behalf of Dyadic and incurred on and after the Effective Date in accordance with the Research Plan and Research Budget. Xxxxxxx will be solely responsible for all internal and external expenses incurred by it in performing the Xxxxxxx Research Activities.
2.7.1 Materials.
(a) Use of Materials. To facilitate the performance of activities under the Research Plan, either Party may provide to the other Party certain biological materials, chemical compounds, proteins, or cell lines owned by or licensed to the supplying Party for use by the other Party as set forth in the Research Plan (such materials, compounds, proteins, or cell lines and any progeny or derivatives thereof, collectively, “Materials”). All Materials shall remain the sole property of the supplying Party, shall be used by the receiving Party solely to perform its obligations under the Research Plan or this Agreement or exercise its rights under this Agreement, shall not be used or delivered to or for the benefit of any Third Party, except pre-approved Subcontractors in the case of Dyadic, without the prior written consent of the supplying Party, and shall not be used in research or testing involving human subjects, unless expressly agreed by the supplying Party. Notwithstanding the foregoing, subject to the other provisions of this Agreement, each Transformed Cell Line as created pursuant to the Research Plan will be (i) considered Materials owned by Xxxxxxx for purposes of this Agreement and (ii) once transferred to Xxxxxxx, will be used by Xxxxxxx and its Affiliates solely to perform its obligations under the Research Plan or this Agreement or exercise its rights under this Agreement, it being expressly understood that Xxxxxxx’x ownership shall not confer any rights to Develop, Manufacture or Commercialize the applicable Xxxxxxx Protein in a C1 Expression System without exercising, as applicable, the Research License Option or one or more Commercial License Options. The Materials supplied under this Section 2.7 (Research Plan; Materials) are supplied “as is” and must be used with prudence and appropriate caution in any experimental work, since not all of their characteristics may be known. Each Party acknowledges that the other Party is providing the Materials for investigational use only in in vitro experiments as further set forth in the Research Plan. Without limiting the foregoing, neither Party will reverse engineer, disassemble, compile, or determine the composition of any Materials Controlled by the other Party and provided to such Party hereunder, except to the extent required in order to perform each Party’s Research Activities or exercise its other rights under this Agreement.
(b) Controls on Use. Each Party will use best efforts to protect and control dissemination of the Materials provided by the other Party or materials that arise from such Materials. This shall include maintaining physical control over such Materials, password protection, access and use records and limitations on dissemination to allow such access and dissemination solely to those specified individuals with a need-to-know who have executed agreements with the applicable Party that contain obligations of confidentiality and non-use at least as restrictive as those set forth in this Agreement.
2.7.2 Supply of Materials by Dyadic. Dyadic shall supply Materials for the Research Activities to the extent set forth in, and in a manner consistent with, the Research Plan, either itself or through one or more Affiliates or Third Parties selected by Dyadic.
2.7.3 Return or Destruction of Materials. Upon expiration or termination of this Agreement, or Xxxxxxx’x earlier request, Dyadic will, at Xxxxxxx’x election, either destroy the Materials owned or supplied by Xxxxxxx and provide Xxxxxxx with written evidence of such destruction or return to such Materials to Xxxxxxx.
2.7.4 Breach Inventions. In the event that either Party uses any of the other Party’s Materials, or other activities conducted, during the Research Term in a manner that is not permitted under the terms of this Agreement, then in such event, all Inventions arising from such activities or use during the Research Term, whether patentable or not (“Breach Inventions”), [***].
2.8 Disclosure of New Technology And Patent Rights. [***].
2.9 Personnel Obligations. Each employee, agent, or independent contractor of a Party or its respective Affiliates or Subcontractors performing work under this Agreement will, prior to commencing such work, be bound by invention assignment obligations, including: (a) promptly reporting any Invention; (b) presently assigning to the applicable Party all of his or her rights, title, and interests in and to any Invention; (c) cooperating in the preparation, filing, prosecution, maintenance, and enforcement of any patent or patent application; and (d) performing all acts and signing, executing, acknowledging, and delivering any and all documents required for effecting the obligations and purposes of this Agreement. It is understood and agreed that any such invention assignment agreement need not reference or be specific to this Agreement.
2.10 Audit Rights. With respect to any facility or site at which a Party, its Affiliates, or Designees conduct Research Activities, each Party shall have the right, during the time period commencing on the Effective Date and lasting until the earlier of (a) the end of the Initial Agreement Term or (b) the completion of the Cell Line Technology Transfer, at its own expense, upon reasonable written notice of no less than five Business Days to the other Party, or such Affiliate or Designee, and during normal business hours, to inspect such site and facility and any records relating thereto once per year (or more frequently for cause), to verify compliance with the terms of this Agreement and Applicable Law in carrying out such Research Activities and its obligations under the Agreement, including, as applicable, GLP, GCP, and GMP. In the event that any such facility or site is found to be non-compliant with such requirements during such audit, and such non-compliance relates to or impacts the activities being conducted by the Parties under this Agreement, the inspected Party will submit a proposed Corrective and Preventative Actions (“CAPA”) within 30 days’ notice of such non-compliance. The inspecting Party shall have the right to review and approve such CAPA prior to implementation thereof by the inspected Party. The inspected Party shall use Commercially Reasonable Efforts to implement such CAPA promptly after approval by the other Party. Failure by the non-compliant Party to resolve the issues identified within 90 days after approval of the CAPA shall constitute a breach of this Agreement. If Dyadic does not have the contractual right to audit any Subcontractor of Dyadic performing Dyadic Research Activities, then it shall not be a breach of this Section 2.10 (Audit Rights) if Dyadic is unable to audit such Subcontractor so long as Dyadic uses Commercially Reasonable Efforts to reach agreement with such Subcontractor to permit audits and implementation of corrective actions by Xxxxxxx under this Section 2.10 (Audit Rights) or if Dyadic conducts such audits and ensures such corrective actions on Xxxxxxx’x behalf.
2.11 Inspections by Regulatory Authorities. Each Party shall, and shall cause its Affiliates and Designees, to cooperate in good faith with respect to Regulatory Authority inspections of any site or facility during the Initial Agreement Term where a Party, its Affiliates, or Designees conduct Research Activities (each an “Inspected Site”). Each Party shall inform the other Party immediately upon receiving notice of such a Regulatory Authority inspection. In the event that any Inspected Site is found to be non-compliant with one or more Applicable Laws, including, as applicable, GLP, GCP, and GMP, and such non-compliance relates to or impacts the Manufacturing process for producing proteins using C1, the inspected Party shall submit to the other Party the inspection report (or equivalent, such as a FDA Form 483) and a proposed CAPA within 30 days after notification of such non-compliance from the relevant Regulatory Authority for review and approval such CAPA. Each Party shall, as applicable, use Commercially Reasonable Efforts to implement such CAPA promptly after approval by the other Party. Failure to resolve the issues identified by the Regulatory Authority within 90 days after approval of the CAPA shall constitute a breach of this Agreement. In addition, Dyadic shall submit the draft response to the applicable Regulatory Authority to Xxxxxxx for review prior to submission thereof and provide Xxxxxxx a minimum of five Business Days for review. For the avoidance of doubt, nothing in this Section 2.11 (Inspections by Regulatory Authorities) is a representation or warranty by Dyadic with respect to whether its facilities or the facilities or activities of its Designees are GLP, GCP or GMP compliant and Dyadic cannot guarantee access by Xxxxxxx to any facilities other than those directly under its control. Each Party’s rights under this Section 2.11 (Inspections by Regulatory Authorities) shall terminate on the earlier of (a) the end of the Initial Agreement Term or (b) the completion of the Cell Line Technology Transfer.
ARTICLE 3
GOVERNANCE
3.1.1 Formation. Within 30 days after the Effective Date, the Parties shall establish a joint governance committee (the “Joint Steering Committee” or “JSC”). The JSC shall consist of two representatives from each of the Parties, each with the requisite experience and seniority to enable such person to make decisions on behalf of the Parties with respect to the issues falling within the jurisdiction of the JSC. From time to time, each Party may substitute one or more of its representatives to the JSC on written notice to the other Party. Xxxxxxx shall select from its representatives the chairperson for the JSC. From time to time, Xxxxxxx may change the representative who shall serve as chairperson on written notice to Dyadic.
3.1.2 Specific Responsibilities. The JSC shall:
(a) oversee and monitor the performance of the Research Activities and address any issues that arise in connection therewith;
(b) review and discuss Data arising from the performance of the activities set forth in the Research Plan;
(c) review and discuss any safety and other issues arising from the performance of the Research Activities;
(d) review, discuss and determine whether to approve any amendments to the Research Plan (including the Research Budget therein);
(e) review, propose modifications to, and approve the Research Budget on a quarterly basis at each JSC meeting, as described in Section 2.1.2 (Research Budget);
(f) discuss the scope, logistics and details regarding the Cell Line Technology Transfer;
(g) in coordination with the JPC, coordinate and monitor the exchange of information between the Parties with respect to the Know-How, Patent Rights, and Technology developed or invented (or, with respect to Patent Rights, Covering or claiming such developments or inventions) in the performance of activities under this Agreement during the Research Term;
(h) establish, oversee, manage and resolve disputes within Working Groups;
(i) establish secure access methods (including secure databases) for each Party to access the Data and the Confidential Information of the other Party; and
(j) perform such other functions as are set forth herein or as the Parties may mutually agree in writing, except where in conflict with any provision of this Agreement.
3.1.3 Meetings and Minutes. The JSC shall meet quarterly during the Research Term, or as otherwise agreed to by the Parties, with the location of such meetings alternating between locations designated by Dyadic and locations designated by Xxxxxxx or by audio or video teleconference if agreed by the Parties. The chairperson of the JSC shall be responsible for calling meetings on no less than 15 Business Days’ notice. Each Party shall make all proposals for agenda items and shall provide all appropriate information with respect to such proposed items at least 10 Business Days in advance of the applicable meeting; provided that under exigent circumstances requiring input by the JSC, a Party may provide its agenda items to the other Party within a shorter period of time in advance of the applicable meeting, or may propose that there not be a specific agenda for a particular meeting, so long as the other Party consents to such later addition of such agenda items or the absence of a specific agenda for such meeting, such consent not to be unreasonably withheld or delayed. The chairperson of the JSC shall prepare and circulate for review and approval of the Parties minutes of each meeting within 30 days after the meeting. The Parties shall agree on the minutes of each meeting promptly, but in no event later than the next meeting of the JSC.
3.1.4 Procedural Rules. The JSC shall have the right to adopt such standing rules as shall be necessary for its work, to the extent that such rules are not inconsistent with this Agreement. A quorum of the JSC shall exist whenever there is present at a meeting at least one representative appointed by each Party. Representatives of the Parties on the JSC may attend a meeting either in person or by telephone, video conference or similar means in which each participant can hear what is said by, and be heard by, the other participants. Representation by proxy shall be allowed. The JSC shall take action by consensus of the representatives present at a meeting at which a quorum exists, with each Party having a single vote irrespective of the number of representatives of such Party in attendance, or by a written resolution signed by at least one representative appointed by each Party. Employees or consultants of either Party that are not representatives of the Parties on the JSC may attend meetings of the JSC; provided that such attendees (a) shall not vote or otherwise participate in the decision-making process of the JSC, and (b) are bound by obligations of confidentiality and non-disclosure equivalent to those set forth in ARTICLE 9 (Confidentiality and Non-Disclosure).
3.1.5 Dispute Resolution.
(a) If the JSC cannot, or does not, reach consensus on an issue at a meeting or within a period of [***] thereafter or such other period as the Parties may agree, then the dispute shall first be referred to the Senior Officers of the Parties, who shall confer in good faith on the resolution of the issue. Any final decision mutually agreed to by the Senior Officers shall be conclusive and binding on the Parties.
(b) If the Senior Officers are not able to agree on the resolution of any such issue within [***] after such issue was first referred to them, then Xxxxxxx, exercising good-faith and subject to the same limitation set out in Section 3.1.6 (Limitations on Authority) shall have final decision-making authority with respect to such issue, except for decisions of the JPC, which will be made and resolved in accordance with Section 3.1.9(c) (Joint Patent Committee). Disputes arising between the Parties in connection with or relating to this Agreement or any document or instrument delivered in connection herewith, and that are outside of the jurisdiction of the JSC, shall be resolved pursuant to ARTICLE 13 (Dispute Resolution).
3.1.6 Limitations on Authority. Each Party shall retain the rights, powers and discretion granted to it under this Agreement and no such rights, powers or discretion shall be delegated to or vested in the JSC unless such delegation or vesting of rights is expressly provided for in this Agreement or the Parties expressly so agree in writing. The JSC shall have no power to amend, modify or waive compliance with this Agreement, which may only be amended or modified as provided in Section 14.7 (Entire Agreement; Amendments) or compliance with which may only be waived as provided in Section 14.10 (Waiver and Non-Exclusive Remedies). The JSC will only have the powers expressly assigned to it in this ARTICLE 3 (Governance) and elsewhere in this Agreement and will not have the authority to decide any issue in a manner that would conflict with the express terms and conditions of this Agreement. In addition, notwithstanding any provision to the contrary set forth in this Agreement, without the other Party’s prior written consent, neither Xxxxxxx (in the exercise of Xxxxxxx’x final decision-making authority), the JSC, the JPC, nor the Senior Officers, in each case, may make a decision that could reasonably be expected to require the other Party to take any action that such other Party reasonably believes would (i) require such other Party to violate any Applicable Law, the requirements of any Regulatory Authority, or any agreement with any Third Party entered into by such other Party or (ii) require such other Party to infringe or misappropriate any Intellectual Property rights of any Third Party. Further, Xxxxxxx will not be permitted, through the exercise of its final decision-making authority, to materially increase Dyadic’s costs or obligations under the then-current Research Plan or allocate additional cost or responsibilities to Dyadic under the Research Plan that are not reimbursed.
3.1.7 Alliance Manager. Each Party shall appoint a person who shall oversee contact between the Parties for all matters between meetings of each JSC and shall have such other responsibilities as the Parties may agree in writing after the Effective Date (each, an “Alliance Manager”). Each Party may replace its Alliance Manager at any time by notice in writing to the other Party. Following the disbandment of the JSC, the Alliance Managers shall continue to act as a liaison between the Parties and shall be responsible for providing and receiving information that is to be exchanged under the terms of this Agreement.
3.1.8 Discontinuation of the JSC. Xxxxxxx may disband the JSC, at its sole discretion, after the expiration of the Research Term and completion of all activities set forth in the Research Plan upon written notice to Dyadic. Once Xxxxxxx has provided such written notice, the JSC shall be terminated and shall have no further rights or obligations under this Agreement, and thereafter, (a) any requirement of a Party to provide information or other materials to the JSC shall be deemed a requirement to provide such information or other materials to the other Party in accordance with the applicable provision of this Agreement and (b) any matters delegated to the JSC shall be made by mutual agreement of the Parties, subject to the dispute resolution provisions under ARTICLE 13 (Dispute Resolution).
3.1.9 Working Groups.
(a) Establishment of Working Groups. From time to time, the JSC may establish and delegate duties to sub-committees or directed teams (each, a “Working Group”) on an “as-needed” basis to oversee particular projects or activities. Each Working Group shall be constituted and shall operate as the JSC determines; provided that each Working Group shall have equal representation from each Party, unless otherwise mutually agreed. Working Groups may be established on an ad hoc basis for purposes of a specific project or on such other basis as the JSC may determine. Each Working Group and its activities shall be subject to the oversight, review and approval of, and shall report to, the JSC. In no event shall the authority of any Working Group exceed that specified for the JSC under this ARTICLE 3 (Governance). All decisions of a Working Group shall be by consensus. Any disagreement between the designees of Xxxxxxx and Dyadic on a Working Group shall be referred to the JSC for resolution.
(b) Specific Working Groups. Within 30 days following the Effective Date, the JSC will establish a Working Group to oversee the performance of Research Activities set forth in work packages 1 and 3 set forth in the Research Plan.
(c) Joint Patent Committee. Within 90 days after the Effective Date, Xxxxxxx and Dyadic shall establish a “Joint Patent Committee” or “JPC” to oversee and coordinate with respect to the continued prosecution of all Patent Rights arising out of or relating to this Agreement. The JPC shall be comprised of one in-house senior patent attorney or a designee from an outside firm from each Party as appointed by such Party. A Party may replace its representative from time to time upon written notice to the other Party. The JPC will serve as the primary contact and forum for discussion between the Parties with respect to intellectual property matters arising under this Agreement, and with respect to the activities set forth in ARTICLE 8 (Intellectual Property Rights). At the JPC a strategy will be discussed with regard to (i) prosecution and maintenance of Dyadic Assigned C1 Rights and Xxxxxxx Assigned Protein Rights, (ii) defense against allegations of infringement of Patent Rights of Third Parties, and (iii) licenses to Patent Rights or Know-How of Third Parties, in each case to the extent such matter would be reasonably likely to have a material impact on this Agreement or the licenses granted hereunder, which strategy will be considered in good faith by the Party entitled to prepare, prosecute, enforce, and defend such Patent Rights, as applicable, hereunder. [***] If the JPC cannot agree on the resolution of a matter for which the JPC is responsible under this Section 3.1.9(c) (Joint Patent Committee) within a period of 30 days, then such matter will be escalated to the JSC for resolution. [***]. Each Party’s representatives on the Joint Patent Committee will consider comments and suggestions made by the other in good faith. Each Party shall bear its own cost of participation on the JPC. Unless otherwise agreed to by the JSC, the JPC shall exist until the termination of the Research Term. After the JPC dissolves, each Party will designate a patent attorney who will be responsible for intellectual property matters under this Agreement and any necessary coordination or consultation under ARTICLE 8 (Intellectual Property Rights).
3.1.10 Expenses. Each Party shall be responsible for all travel and related costs and expenses for its members and other representatives to attend meetings of, and otherwise participate on, the JSC, the JPC or other Working Groups.
ARTICLE 4
TARGET RESERVATION; OPTIONS AND OPTION EXERCISE
4.1.1 Initial Reserved Targets. [***].
4.1.2 Selection of Additional Targets; Replacement of Targets.
(a) Selection of Additional Targets. [***].
(b) Target Reservation Process. [***]
(c) Occupied Targets[***].
(d) Gatekeeper. [***].
(e) C1 Biological Target List. [***].
(f) Expiration of Pre-Existing Restrictions; Release of Targets. [***]
(g) Identification of Targets. [***].
4.2 Xxxxxxx Options.
4.2.1 Research License Option.
(a) Grant of Research License Option. Effective as of the Effective Date, Dyadic hereby grants, and shall cause its Affiliates to grant, to Xxxxxxx an option to obtain the Xxxxxxx Research License (the “Research License Option”).
(b) Exercise of Research License Option. Xxxxxxx may, in its sole discretion, exercise the Research License Option at any time prior to the expiration of the Initial Agreement Term, by providing written notice of such exercise to Dyadic (the date of delivery of such notice, the “Research License Option Exercise Date”). Promptly following Dyadic’s receipt of such written notice, Dyadic shall provide Xxxxxxx with an updated disclosure schedule with respect to Dyadic’s representations and warranties set forth in Schedule 10.2 (Dyadic Disclosure Schedule). If Xxxxxxx exercises the Research License Option then (i) Dyadic will grant the license to Xxxxxxx set forth in Section 5.2 (Xxxxxxx Research License), (ii) Xxxxxxx will pay the Research License Option Exercise Fee to Dyadic in accordance with Section 7.3.1 (Research License Option Exercise Fee), and (iii) Dyadic will perform the Cell Line Technology Transfer to Xxxxxxx as required under Section 4.2.1(c) (Cell Line Technology Transfer).
(c) Obligation to Perform Cell Line Technology Transfer. Following the Research License Option Exercise Date, Dyadic will perform the Cell Line Technology Transfer in accordance with Section 6.1.4 (Cell Line Technology Transfer).
4.2.2 Reserved Targets Commercial License Options.
(a) Reserved Targets Option. [***].
(b) Exercise of Commercial License Options. Xxxxxxx may, at any time during the Term prior to the filing of an IND with respect to a Xxxxxxx Protein directed to one or more Reserved Targets, in its sole discretion, exercise a Commercial License Option with respect to the Program for such Xxxxxxx Protein directed to such Reserved Target(s), by providing written notice of such exercise to Dyadic, which notice will identify the applicable Xxxxxxx Protein and corresponding Reserved Target for such Program (the date of delivery of such notice, the “Program Exercise Date”). On a Program-by-Program basis, if Xxxxxxx exercises a Commercial License Option, then (i) Xxxxxxx will pay the Program Exercise Fee for such Program to Dyadic in accordance with Section 7.3.2 (Program Exercise Fees For Commercial License Grants), and (ii) Dyadic will grant the license to Xxxxxxx set forth in Section 5.3 (Xxxxxxx Commercial Licenses) with respect to such Program.
4.2.3 Exclusive Target Option.
(a) Election to Obtain Exclusive Target Option. During the Initial Agreement Term, Xxxxxxx will have the right to elect to obtain an option to take the Exclusive Target License (the “Exclusive Target Option”). Xxxxxxx may, in its sole discretion, elect to obtain such Exclusive Target Option by providing written notice thereof to Dyadic together with the payment of the Exclusive Target Option Grant Fee in accordance with Section 7.3.4(a) (Exclusive Target Option Grant Fee) (the date of delivery of such notice, the “Exclusive Target Option Grant Date”) and as of such date, Dyadic will and hereby does grant, and will cause its Affiliates to so grant, the Exclusive Target Option to Xxxxxxx, which option shall last until two years following such Exclusive Target Option Grant Date (the “Exclusive Target Option Period”). In furtherance of the foregoing option grant, during the Exclusive Target Option Period, Dyadic will not, and will not enable or grant any rights to any Affiliate or Third Party to, Develop, license, or use the C1 Platform to produce any protein directed to the Xxxxxxx Exclusive Target. If, during the Initial Agreement Term, Xxxxxxx has not yet elected to take the Exclusive Target Option and a Third Party makes a bona fide offer to Dyadic to Develop or license the C1 Platform to produce a protein directed to the Xxxxxxx Exclusive Target, then Dyadic will provide Xxxxxxx with prompt written notice, and Xxxxxxx will have [***] from the receipt of such notice to exercise its right to obtain the Exclusive Target Option.
(b) Exercise of Exclusive Target Option. Except in the event that Xxxxxxx has failed to exercise its right to obtain the Exclusive Target Option [***] following receipt of written notice of an applicable bona fide Third Party offer pursuant to Section 4.2.3(a) (Election to Obtain Exclusive Target Option), Xxxxxxx may, at any time during the Exclusive Target Option Period, in its sole discretion, exercise the Exclusive Target Option by providing written notice thereof to Dyadic together with the Exclusive Target Option Exercise Fee in accordance with Section 7.3.4(b) (Exclusive Target Option Exercise Fee) (the date of delivery of such notice, the “Exclusive Target Option Exercise Date”). If Xxxxxxx exercises the Exclusive Target Option, then Dyadic will grant to Xxxxxxx the Exclusive Target License under Section 5.4 (Xxxxxxx Exclusive Target License) and Xxxxxxx may exercise the Commercial License Option for a Program for the Xxxxxxx C1 Protein that is directed to the Xxxxxxx Exclusive Target.
4.3 Effects of Failure to Exercise Research License Option. If Xxxxxxx does not exercise the Research License Option by the end of the Initial Agreement Term, then this Agreement will terminate in its entirety, and the effects of termination set forth in Section 12.7.1 (Termination in Entirety) will apply.
ARTICLE 5 LICENSE GRANTS
5.1.1 Xxxxxxx Research Plan License. [***].
5.1.2 Dyadic Research Plan License. [***].
5.2 Xxxxxxx Research License. [***].
5.3 Xxxxxxx Commercial Licenses. Effective on the Effective Date, and subject to the remainder of this Section 5.3 (Xxxxxxx Commercial License), Dyadic, on behalf of itself and its Affiliates, hereby grants to Xxxxxxx and its Affiliates [***] under the Licensed Intellectual Property and Dyadic’s and its Affiliates’ interests in the Jointly Owned Rights to use the C1 Platform to Manufacture, use, Develop, import, export and Commercialize a maximum of [***] each directed to Reserved Targets during the Term and in the Field, [***], which license will be exclusive with respect to the sequence of such Xxxxxxx Proteins and non-exclusive (subject to Section 4.2.3 (Exclusive Option Target)) with respect to (a) the Targets to which such Xxxxxxx Proteins are directed and (b)[***] (each a “Xxxxxxx Commercial License”). Xxxxxxx and its Affiliates will not have the right to practice the Xxxxxxx Commercial Licenses unless and until, (i) on a Program-by-Program basis, the occurrence of the Program Exercise Date for the Program for such Xxxxxxx Protein that is directed to such Reserved Target, or (ii) the Program Exercise Date has not occurred with respect to Xxxxxxx Proteins directed to one or more Reserved Targets and this Agreement is rejected by or on behalf of Dyadic pursuant to the Bankruptcy Code or is repudiated by or on behalf of Dyadic under the Bankruptcy Code or other Applicable Laws, provided that Xxxxxxx pays or has paid the Research License Option Exercise Fee and the Program Exercise Fee for each Program in connection with which Xxxxxxx intends to practice a Xxxxxxx Commercial License and Xxxxxxx and its Designees are, at such time, in material compliance with this Agreement. Any exercise of rights under the Xxxxxxx Commercial Licenses by Xxxxxxx after such a rejection or repudiation will be subject to and in accordance with the Bankruptcy Code, including Section 365(n).
5.4 Xxxxxxx Exclusive Target License. Effective on the Effective Date, and subject to the remainder of this Section 5.4 (Xxxxxxx Exclusive Target License), Dyadic, on behalf of itself and its Affiliates, hereby grants to Xxxxxxx and its Affiliates an exclusive (including with regard to Dyadic and its Affiliates), worldwide license under the Licensed Intellectual Property to use the C1 Platform to Manufacture any and all proteins directed to the Xxxxxxx Exclusive Target for commercial use in the Territory[***] (the “Exclusive Target License”). Xxxxxxx and its Affiliates will not have the right to practice the Exclusive Target License unless and until (a) the occurrence of the Exclusive Target Option Exercise Date, or (b) if the Exclusive Target Option Exercise Date has not occurred and this Agreement is rejected by or on behalf of Dyadic pursuant to the Bankruptcy Code or is repudiated by or on behalf of Dyadic under the Bankruptcy Code or other Applicable Laws, provided that Xxxxxxx pays or has paid the Exclusive Target Option Grant Fee and the Exclusive Target Option Exercise Fee. Any exercise of rights under the Xxxxxxx Commercial Licenses by Xxxxxxx after such a rejection or repudiation will be subject to and in accordance with the Bankruptcy Code, including Section 365(n).
5.5 Unblocking Licenses.
5.5.1 Dyadic to Xxxxxxx. [***].
5.5.2 Xxxxxxx to Dyadic. [***].
5.6 Sublicenses.
5.6.1 Sublicensing by Xxxxxxx.
(a) Right to Sublicense. [***].
(b) Danisco Sublicense Requirements. [***].
5.6.2 Sublicensing by Dyadic. [***].
5.7 Use of Affiliates and Contractors and Subcontractors. Each Party may perform any or all of its obligations or exercise any or all of its rights under this Agreement, through one or more of its Affiliates or Subcontractors; provided, in each case, that: (a) none of the other Party’s rights hereunder are diminished or otherwise adversely affected as a result of such delegation or contracting; (b) each such Affiliate or Third Party contractor, undertakes in writing obligations of confidentiality and non-use regarding Confidential Information of the other Party that are at least as stringent as those undertaken by such Party pursuant to ARTICLE 9 (Confidentiality and Non-Disclosure); (c) each such Affiliate and Subcontractor of a Party agrees in writing to assign to such Party any and all Inventions developed or invented by such Affiliate or Subcontractor in the course of performing the delegated or contracted activities, as necessary for such Party to comply with its obligations under Section 8.1 (Ownership of Intellectual Property Rights); and (d) such Party shall at all times be fully responsible for the performance of such Affiliate or Subcontractor and the compliance of such Affiliate or Subcontractor with this Agreement; and provided further that Dyadic will obtain Xxxxxxx’x prior written consent before performing any of its obligations under this Agreement through a Subcontractor other than a Subcontractor listed on Schedule 5.7 (Approved Subcontractors).
5.8 Xxxxxxx Covenant. Xxxxxxx will not use the C1 Platform to undertake any activities other than activities conducted for Xxxxxxx, its Affiliates, its Subcontractors, or Permitted Representatives.
5.9 Know-How And Technology Transfer.
5.9.1 Initial Technology Transfer: Specification. Annexed hereto as Schedule 5.9.1 (Initial Technology Transfer: Specification) is a preliminary specification of Dyadic-Controlled Know-How and Technology included in the Licensed Intellectual Property that, as of the Effective Date and in the reasonable judgment of the Parties, is necessary for Xxxxxxx and its Affiliates to (a) exercise the license granted to Xxxxxxx and its Affiliates under Section 5.1 (Research Plan License) in accordance with this Agreement; and (b) otherwise exercise Xxxxxxx’x rights and perform Xxxxxxx’x obligations under the Research Plan. In accordance with the Research Plan, Dyadic will provide for the transfer of such items of Dyadic-Controlled Know-How and Technology, including those Transformed Cell Lines provided for under the Research Plan, in a manner as required by and consistent with the Research Plan. The Parties acknowledge that Schedule 5.9.1 (Initial Technology Transfer: Specification) may be incomplete. Xxxxxxx’x Alliance Manager will provide on a regular basis, but not more often than once every 14 days for up to three months following the initial transfer of the Know-How and Technology set forth on Schedule 5.9.1 (Initial Technology Transfer: Specification) (the “Evaluation Period”), to the Dyadic Alliance Manager an itemized written list of any additional Dyadic Controlled Know-How and Technology it seeks or the manners in which it believes the transfer is not complete for its intended purpose (including a written specification of the activities undertaken to confirm the suitability and operability of the Dyadic Controlled Know-How and Technology provided for under this Section 5.9 (Know-How and Technology Transfer)). Upon the receipt of any such itemized list, Dyadic will use Commercially Reasonable Efforts to resolve any items so specified and to effectuate a transfer of any missing Dyadic-Controlled Know-How or Technology directly or through applicable Third Party Subcontractors. At the end of the Evaluation Period, the Xxxxxxx Alliance Manager will generate a final list, [***]. All such transfers under this Section 5.9.1 (Initial Technology Transfer: Specification) shall be as “as is” and without any warranties except as expressly provided for herein. Dyadic’s obligations under this Section 5.9.1 (Initial Technology Transfer: Specification) shall cease following the Successful Cell Line Transfer.
5.9.2 Follow On Transfer. On a regular basis, through the JSC and the Alliance Managers, Dyadic shall provide information on additional Dyadic Controlled Know-How and Technology that Dyadic in its reasonable judgment believes are or would be necessary to Xxxxxxx or its Affiliates. From time to time, but not more often than once per Calendar Quarter commencing on the first Calendar Quarter after the end of the Evaluation Period, Xxxxxxx may make supplemental requests to Dyadic seeking additional Dyadic Controlled Know-How and Technology it believes are in the Control of Dyadic and are necessary for Xxxxxxx and its Affiliates to exercise their rights under this Agreement. Xxxxxxx shall make all such requests in writing with a specification of its need and intended use of the Dyadic Controlled Know-How and Technology to be transferred. Dyadic will use Commercially Reasonable Efforts to effectuate such a transfer directly or through applicable Third Party Subcontractors. All such transfers under this Section 5.9.2 (Follow On Transfer) shall be as “as is” and without any warranties except as expressly provided for herein.
5.9.3 Technical Assistance. In addition to providing the Know-How and Technology that is part of the Licensed Intellectual Property to Xxxxxxx in accordance with this Section 5.9 (Know-How and Technology Transfer), Dyadic and its personnel will provide Xxxxxxx with reasonable technical assistance at Xxxxxxx’x reasonable request ([***], at the applicable FTE Rate per FTE based on the number of FTEs of Dyadic actually used to provide such assistance, provided, however, that Xxxxxxx will reimburse Dyadic for any out-of-pocket costs incurred by Dyadic arising from the provision of such technical assistance by a Third Party regardless of the timing of the incurrence of such activities.
5.10 In‑License Agreements.
5.10.1 Existing In-Licenses. [***].
5.10.2 New In-License Agreements. [***].
ARTICLE 6
XXXXXXX PROGRAMS
6.1 Development under Research License.
6.1.1 General. Following the Research License Option Exercise Date, subject to the terms of this Agreement, Xxxxxxx (itself or through its Affiliates, Subcontractors, or Permitted Representatives) shall have sole authority over the Manufacture, use, Development, import and export of Xxxxxxx Proteins (regardless of Target) during the Term and in the Field using the C1 Platform, including all IND-Enabling Studies and CMC Development with respect to Xxxxxxx C1 Proteins. At Xxxxxxx’x request, Dyadic shall provide reasonable assistance to Xxxxxxx in connection with such activities, including by providing Xxxxxxx with any Know-How or Technology within the Licensed Intellectual Property that has not been previously transferred to Xxxxxxx that would be necessary for such activities or which was used as part of the Research Plan, [***], at the applicable FTE Rate based on the number of FTEs of Dyadic actually used to provide such assistance, provided, however, that Xxxxxxx will reimburse Dyadic for any out-of-pocket costs incurred by Dyadic arising from the provision of such assistance by a Third Party regardless of the timing of the incurrence of such activities.
6.1.2 Regulatory Matters. [***].
6.1.3 Safety Data. Dyadic shall report to Xxxxxxx by written notice within five days of receiving a report of any adverse events that occur during Clinical Trials related to the use of the C1 Platform in Manufacturing of therapeutic proteins, in order to monitor the safety of any proteins produced using the C1 Platform.
6.1.4 Cell Line Technology Transfer.
(a) Research Transfer. [***].
(b) Cell Line Technology Transfer Procedures and Assistance. Without limiting the foregoing, in connection with the Cell Line Technology Transfer and during the time period commencing on the Research License Option Exercise Date and until Successful Cell Line Transfer is achieved:
(i) Dyadic shall provide, and shall use Commercially Reasonable Efforts to cause its Third Party manufacturers to provide (including by using Commercially Reasonable Efforts to negotiate contractual obligations for such Third Party manufacturers to do so under agreements entered into following the Effective Date), all reasonable assistance requested by Xxxxxxx to enable Xxxxxxx (or its Affiliate or designated Subcontractor, as applicable) to implement the Process. If requested by Xxxxxxx, such assistance shall include facilitating the entering into of agreements with applicable Third Parties relating to the C1 Platform or the Process;
(ii) Dyadic shall make available, and shall use Commercially Reasonable Efforts to cause its Third Party manufacturers to make available (including by using Commercially Reasonable Efforts to negotiate contractual obligations for such Third Party manufacturers to do so under agreements entered into following the Effective Date), to Xxxxxxx (or its Affiliate or designated Third Party manufacturer, as applicable) from time to time as Xxxxxxx may request, all Process-related Know-How and Technology, information and materials relating to the Process, and all documentation constituting material support, performance advice, shop practice, standard operating procedures, specifications as to materials to be used and control methods, that are necessary to enable Xxxxxxx (or its Affiliate, Subcontractor, or Permitted Representative, as applicable) to use and practice the Process;
(iii) Dyadic will transfer to Xxxxxxx (A) [***], (B) all information and reagents necessary to create expression cell lines for Xxxxxxx Proteins of Xxxxxxx’x choosing, (C) all information on the parental cell line Development history necessary prepare and file Regulatory Documentation and applications for Regulatory Approval for Xxxxxxx C1 Proteins, including description of plasmids, cloning method descriptions, media and growth conditions used during cell line Development and cell banking, cell line testing reports, and certifications that cell lines were created without use of animal-derived materials, and (D) fermentation process description, process controls, media and feeds used by Dyadic for protein production using the C1 Platform;
(iv) Dyadic shall cause all appropriate employees and representatives of Dyadic and its Affiliates to meet with, and shall use Commercially Reasonable Efforts to cause all appropriate employees and representatives of its Third Party manufacturers to meet with (including by using Commercially Reasonable Efforts to negotiate contractual obligations for such Third Party manufacturers to do so under agreements entered into following the Effective Date), employees or representatives of Xxxxxxx (or its Affiliate or designated Third Party manufacturer, as applicable) at the applicable manufacturing facility at mutually convenient times to assist with the working up and use of the Process and with the training of the personnel of Xxxxxxx (or its Affiliate, Subcontractor, or Permitted Representative, as applicable) to the extent necessary or reasonably useful to enable Xxxxxxx (or its Affiliate, Subcontractor, or Permitted Representative, as applicable) to use and practice the Process;
(v) Without limiting the generality of clause (B) above, Dyadic shall cause all appropriate analytical and quality control laboratory employees and representatives of Dyadic and its Affiliates to meet with, and shall use Commercially Reasonable Efforts to cause all appropriate analytical and quality control employees and representatives of its Third Party manufacturers to meet with (including by using commercially reasonable efforts to negotiate contractual obligations for such Third Party manufacturers to do so under agreements entered into following the Effective Date), employees or representatives of Xxxxxxx (or its Affiliate, Subcontractor, or Permitted Representative, as applicable) at the applicable manufacturing facility and make available all necessary equipment, at mutually convenient times, to support and execute the transfer of all applicable analytical methods and the validation thereof (including all applicable Dyadic Background Technology, Dyadic Owned Rights, methods, validation documents and other documentation, materials and sufficient supplies of all primary and other reference standards);
(vi) Dyadic shall take such steps, and shall use Commercially Reasonable Efforts to cause its Third Party manufacturers to take such steps (including by using Commercially Reasonable Efforts to negotiate contractual obligations for such Third Party manufacturers to do so under agreements entered into following the Effective Date), as are necessary to assist in reasonable respects Xxxxxxx (or its Affiliate, Subcontractor, or Permitted Representative, as applicable) in obtaining any necessary licenses, permits or approvals from Regulatory Authorities with respect to the use of the Process to produce Xxxxxxx C1 Proteins at the applicable facilities; and
(vii) Dyadic shall provide, and shall use Commercially Reasonable Efforts to cause its Third Party manufacturers to provide (including by using Commercially Reasonable Efforts to negotiate contractual obligations for such Third Party manufacturers to do so under agreements entered into following the Effective Date), such other assistance as Xxxxxxx (or its Affiliate or designated Third Party manufacturer, as applicable) may reasonably request to enable Xxxxxxx (or its Affiliate or designated Third Party manufacturer, as applicable) to use and practice the Process and otherwise to Manufacture Xxxxxxx Proteins using the C1 Platform.
(c) Manufacturing Transfer. [***].
(d) Ongoing Assistance. [***].
6.1.5 Manufacturing. During the Term following the Research License Option Exercise Date and the completion of the Cell Line Technology Transfer, Xxxxxxx (itself or through its Affiliates, Subcontractors, or Permitted Representatives) shall be responsible for, at its sole cost and expense, the Manufacture and supply of the Xxxxxxx C1 Proteins necessary to Manufacture, use, Develop, import and export Xxxxxxx C1 Proteins under Xxxxxxx’x rights under the Xxxxxxx Research License.
6.2 Commercial Programs. Effective on a Program-by-Program basis upon the Program Exercise Date for such Program, during the Term:
6.2.1 General. Xxxxxxx (itself or through its Affiliates, Subcontractors, or Permitted Representatives) shall have sole authority over the Manufacture, use, Development, import, export and Commercialization of the Xxxxxxx C1 Protein for such Program during the Term and in the Field.
6.2.2 Regulatory Matters.
(a) Xxxxxxx (itself or through its Affiliates, Subcontractors, or Permitted Representatives) shall be solely responsible for, at its sole cost and expense, preparing, filing and maintaining all Regulatory Documentation (including all INDs, Marketing Approval Applications, Regulatory Approvals and Product Labeling) with respect to one or more Xxxxxxx C1 Proteins and interacting and communicating with Regulatory Authorities with respect thereto for the applicable Xxxxxxx C1 Protein in the Field in the Territory.
(b) All Regulatory Documentation (including all INDs, Marketing Approval Applications, Regulatory Approvals and Product Labeling) relating to the applicable Xxxxxxx C1 Protein in the Field in the Territory shall be owned by, and shall be the sole property and held in the name of, Xxxxxxx or its designated Affiliate, or designee. If requested by a Regulatory Authority, then Xxxxxxx and its Affiliates will provide Dyadic or such Regulatory Authority (as applicable based on such request) with access to and the right to review Regulatory Documentation relating to Xxxxxxx C1 Proteins.
(c) Dyadic, shall use Commercially Reasonable Efforts to support Xxxxxxx and its Affiliates as may be reasonably necessary or appropriate, in obtaining all Regulatory Approvals for the applicable Xxxxxxx C1 Protein, including providing necessary documents, data, or other materials required by Applicable Law to obtain all Regulatory Approvals and Xxxxxxx will reimburse Dyadic for such assistance at the applicable FTE Rate.
6.2.3 Manufacturing Activities. During the Term following the Program Exercise Date Xxxxxxx (itself or through its Affiliates, Subcontractors, or Permitted Representatives) shall be responsible for, at its sole cost and expense, the Manufacture and supply of the Xxxxxxx C1 Proteins necessary to perform Development and Commercialization of Xxxxxxx C1 Proteins under this Agreement.
6.3 Xxxxxxx Commercialization. Effective on a Program-by-Program basis upon the Program Exercise Date for such Program, during the Term:
6.3.1 Commercialization Activities. Xxxxxxx (itself or through its Affiliates, Subcontractors or Permitted Representatives) shall be solely responsible for, at its sole cost and expense, the Commercialization of the Xxxxxxx C1 Protein for such Program in the Field in the Territory.
6.3.2 Booking of Sales; Distribution. Xxxxxxx (itself or through its Affiliates, Subcontractors or Permitted Representatives) shall be solely responsible for, at its sole cost and expense, invoicing and booking sales, establishing all terms of sale (including pricing and discounts) and warehousing, and distributing the Xxxxxxx C1 Protein for such Program in the Territory and to perform or cause to be performed all related services. Xxxxxxx shall handle all returns, recalls or withdrawals, order processing, invoicing, collection, distribution and inventory management with respect to the Xxxxxxx C1 Protein for such Program in the Territory.
6.3.3 Product Trademarks. Xxxxxxx shall be solely responsible for, at its sole cost and expense, determining the Product Trademarks to be used with respect to the Exploitation of the Xxxxxxx C1 Protein for such Program on a worldwide basis and Xxxxxxx shall own such Product Trademarks.
6.4.1 [***].
6.4.2 Termination of this Agreement in accordance with Section 12.2 (Termination for Material Breach) will be Dyadic’s sole remedy for breach by Xxxxxxx of this Section 6.4 (Xxxxxxx Diligence).
6.5 Compliance with Applicable Law. Xxxxxxx shall perform its activities under this ARTICLE 6 (Xxxxxxx Programs) in compliance with all Applicable Law.
6.6 Books and Records. Xxxxxxx and its Affiliates, as well as any Designee or any other Third Party who receives the benefit of this Agreement or access to any Licensed Intellectual Property shall keep complete and accurate records of all such use and, as applicable, its compliance with the terms and conditions of this Agreement.
ARTICLE 7
PAYMENTS
7.1 Initial Target Reservation Fee. Within [***] of the Effective Date, Xxxxxxx shall pay to Dyadic a non-refundable, non-creditable payment in the amount of $500,000 in consideration for the rights granted to Xxxxxxx under Section 4.1.1 (Initial Reserved Targets) and as consideration for Development activities carried out by Dyadic with respect to the C1 Platform prior to the Effective Date.
7.2 Research Plan Costs and Expenses. Xxxxxxx will be responsible for all FTE Costs and Out-of-Pocket Expenses incurred by Dyadic in the performance of the Dyadic Research Activities during the Research Term to the extent within the Research Budget that was approved by the JSC (the “Reimbursable Research Costs”). Within [***] after the end of each Calendar Quarter during the Research Term, Dyadic shall provide a written report to Xxxxxxx setting forth in reasonable detail the Reimbursable Research Costs incurred by Dyadic during such Calendar Quarter, including the type and extent of Dyadic Research Activities to which such Reimbursable Research Costs are attributable, invoices from Third Party vendors or contractors for Out-of-Pocket Expenses, the number of Dyadic FTEs devoted to such Dyadic Research Activities, and a written invoice for such Reimbursable Research Costs. Xxxxxxx shall have [***] to review such report and request reasonable additional information related to the FTE Costs and Out-of-Pocket Expenses incurred by Dyadic under the Research Plan during such Calendar Quarter in order to confirm that spending is in conformance with the approved Research Budget. If Dyadic has provided the applicable report and invoice on time in accordance with this Section 7.2 (Research Plan Costs and Expenses), then Xxxxxxx shall pay (in Euros) undisputed Reimbursable Research Costs within [***] after receipt of the applicable written invoice for such Reimbursable Research Costs. If, in any Calendar Quarter, Dyadic incurs aggregate FTE Costs and Out-Of-Pocket Expenses in excess of the amount set forth in the Research Budget for the Dyadic Research Activities performed in such Calendar Quarter, Xxxxxxx shall not be obligated to reimburse such excess FTE Costs or Out-Of-Pocket Expenses except to the extent the JSC approves such excess FTE Costs or Out-of-Pocket Expenses pursuant to an amendment to the Research Budget approved in accordance with Section 2.1.3 (Amendments to Research Plan) before such costs are incurred. Dyadic shall provide to Xxxxxxx the estimated Reimbursable Research Costs for each applicable Calendar Quarter within [***] prior to the end of such Calendar Quarter to enable Xxxxxxx to appropriately accrue the Reimbursable Research Costs. Such estimate shall include the Reimbursable Research Costs incurred in such Calendar Quarter to date and an estimate of the Reimbursable Research Costs for the remainder of such Calendar Quarter.
7.3 Option Exercise Fees.
7.3.1 Research License Option Exercise Fee. In consideration for the exercise of the Research License Option and grant of the Xxxxxxx Research License to Xxxxxxx and the other rights triggered thereby, Xxxxxxx will pay to Dyadic a one-time option exercise fee of [***] (the “Research License Option Exercise Fee”) within [***] following Xxxxxxx’x delivery of written notice of its decision to exercise the Research License Option pursuant to Section 4.2.1(b) (Exercise of Research License Option) and receipt of an email invoice therefor from Dyadic. Dyadic will issue an email invoice to Xxxxxxx for the Research License Option Exercise Fee immediately upon receipt of such written notice from Xxxxxxx and a formal invoice within [***] of such receipt.
7.3.2 Cell Line Transfer Fee. Pursuant to Section 6.1.4 (Cell Line Technology Transfer), in consideration of achievement of Successful Cell Line Transfer, Xxxxxxx will pay to Dyadic a one-time technology transfer fee of [***] (the “Cell Line Technology Transfer Fee”) within [***] following Xxxxxxx’x delivery of written notice of such Successful Cell Line Transfer pursuant to Section 6.1.4(a) (Research Transfer) and receipt of an email invoice therefor from Dyadic. Dyadic will issue an email invoice to Xxxxxxx for the Cell Line Technology Transfer Fee immediately upon receipt of such written notice from Xxxxxxx and a formal invoice within [***] of such receipt.
7.3.3 Program Exercise Fees For Commercial License Grants. On a Program-by-Program basis, in consideration for the exercise of the Commercial License Option and grant of the Xxxxxxx Commercial License for such Program to Xxxxxxx, Xxxxxxx will pay to Dyadic an option exercise fee of [***] (each a “Program Exercise Fee”) within [***] following Xxxxxxx’x delivery of written notice of its decision to exercise the Commercial License Option for a particular Program pursuant to Section 4.2.2(b) (Exercise of Commercial License Options) and receipt of an email invoice therefor from Dyadic. Dyadic will issue an email invoice to Xxxxxxx for the Program Exercise Fee immediately upon receipt of such written notice from Xxxxxxx and a formal invoice within [***] of such receipt.
7.3.4 Exclusive Target Fees.
(a) Exclusive Target Option Grant Fee. In consideration for the grant of the Exclusive Target Option, Xxxxxxx will pay to Dyadic [***] (the “Exclusive Target Option Grant Fee”) within [***] following Xxxxxxx’x delivery of written notice of its election to obtain the Exclusive Target Option pursuant to Section 4.2.3(a) (Exclusive Target Option Grant) and receipt of an email invoice therefor from Dyadic. Dyadic will issue an email invoice to Xxxxxxx for the Exclusive Target Option Grant Fee immediately upon receipt of such written notice from Xxxxxxx and a formal invoice within [***] of such receipt.
(b) Exclusive Target Option Exercise Fee. In consideration for the exercise of the Exclusive Target Option and grant of the Exclusive Target License to Xxxxxxx, Xxxxxxx will pay to Dyadic [***] (the “Exclusive Target Option Exercise Fee”) within [***] following Xxxxxxx’x delivery of written notice of its election to exercise the Exclusive Target Option pursuant to Section 4.2.3(b) (Exercise of Exclusive Target Option) and receipt of an email invoice therefor from Dyadic. Dyadic will issue an email invoice to Xxxxxxx for the Exclusive Target Option Exercise Fee immediately upon receipt of such written notice from Xxxxxxx and a formal invoice within [***] of such receipt.
7.4 Development and Commercial Milestones by Xxxxxxx. In partial consideration of the rights granted by Dyadic to Xxxxxxx hereunder and subject to the terms and conditions set forth in this Agreement, Xxxxxxx shall pay to Dyadic the amounts set forth below within [***]following (i) Xxxxxxx’x delivery of written notice of the first achievement of each of the following milestone events by Xxxxxxx, or its Affiliate with respect to a Xxxxxxx C1 Protein that is the subject of a Program for which Xxxxxxx exercised the Commercial License Option hereunder following the applicable Program Exercise Date and (ii) receipt of an email invoice therefor from Dyadic. Xxxxxxx will deliver such written notice of achievement to Dyadic within [***]following the Calendar Quarter of such achievement, and Dyadic will issue an email invoice to Xxxxxxx for the applicable milestone payment immediately upon receipt of such written notice from Xxxxxxx and a formal invoice within [***] of such receipt.
|
Development Milestone |
Development Payment |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
Each milestone payment in this Section 7.4 (Development and Commercial Milestones by Xxxxxxx) shall be payable only once per Program for which Xxxxxxx has exercised the Commercial License Option upon the first achievement of such milestone by the first Xxxxxxx C1 Protein of such Program and no amounts shall be due for subsequent or repeated achievements of such milestone by Xxxxxxx C1 Proteins that are the subject of the same Program.
7.5 Production-Based Milestones.
7.5.1 Determination of Titers For Each Xxxxxxx C1 Protein. [***]
7.5.2 Production-Based Milestone Payments. In partial consideration of the rights granted by Dyadic to Xxxxxxx hereunder, [***], a “Production-Based Milestone Payment”) in the corresponding amount set forth in the right-hand column of the table below. Each such milestone payment shall be due within [***] following the end of the Calendar Quarter of when such milestone was achieved.
|
Cumulative Gross Mass of Xxxxxxx C1 Protein with Accessible Titers greater than or equal to [***] and less than [***] |
Milestone Payment Amount |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
Notwithstanding anything contained in Section 7.5 (Production-Based Milestones), each milestone payment in this Section 7.5 (Production-Based Milestones) shall be payable only once per Xxxxxxx C1 Protein that is the subject of a Program for which Xxxxxxx has exercised the Commercial License Option upon the first achievement of such milestone during the Program Payment Term, and no amounts shall be due for subsequent or repeated achievements of such milestone for Xxxxxxx C1 Proteins that are the subject of the same Program. The maximum aggregate amount payable by Xxxxxxx pursuant to this Section 7.5 (Production-Based Milestones) is $[***] with respect to which Accessible Titers greater than [***] is achieved.
7.5.3 Milestone Payment Reductions. [***].
7.5.4 Milestone Payment Increases. [***]
7.5.5 Multi-Specific Antibodies Adjustments. Notwithstanding any provision to the contrary set forth in this Section 7.5 (Production-Based Milestones), for [***]:
(a) Milestone Payment Reductions. (i) If a multi-specific Xxxxxxx C1 Protein has Accessible Titers less than [***];
(b) Milestone Payment Baseline Adjustment. [***];
(c) Milestone Payment Increases. [***].
7.5.6 Expiration of Program Payment Term. [***].
7.6 Third Party IP.
7.6.1 Third Party IP Existing as of the Effective Date. [***].
7.6.2 Third Party IP Existing as of the Cell Line Technology Transfer. [***].
7.6.3 Limitations on Third Party IP Rights. [***].
7.7 Mode of Payment; Offsets. All payments to Dyadic under this Agreement shall be made by electronic funds transfer in US Dollars in the requisite amount to such bank account as Dyadic has established with Xxxxxxx & Xxxxxxx Accounts Payable supplier portal at xxx.xx.xxx.xxx. Janssen shall have the right to offset any payment that is owed by Dyadic but not paid against any payments owed by Xxxxxxx, if any, under this Agreement.
7.8 Income Tax Withholding. Xxxxxxx shall make all payments under this Agreement without deduction or withholding for Taxes except to the extent that any such deduction or withholding is required by law in effect at the time of payment; provided, however, that Xxxxxxx shall use reasonable, good faith efforts to give Dyadic advance notice of its intention to make such deduction or withholding. Any Tax required to be withheld on amounts payable under this Agreement will be timely paid by Xxxxxxx on behalf of Dyadic to the appropriate Governmental Authority, and Xxxxxxx will furnish Dyadic with proof of payment of such Tax as well as any official receipts issued by the applicable Governmental Authority or other evidence as is reasonably requested to establish that such Taxes have been paid. Any such Tax required to be withheld will be an expense of and borne by Dyadic. If any such Tax is assessed against and paid by Xxxxxxx, then Dyadic will indemnify and hold harmless Xxxxxxx from and against such Tax. The Parties shall cooperate with respect to all documentation required by any taxing authority or reasonably requested to secure a reduction in the rate of applicable withholding taxes, and the Parties shall provide reasonable mutual assistance with respect to any claim of refund or exemption from Taxes under any relevant agreement or treaty. On the Effective Date, Dyadic will deliver to Xxxxxxx an accurate and complete Internal Revenue Service Form W-9 and as soon as practicable thereafter, an exemption certificate under the respective treaty between the United States and Germany. The Parties hereby provide their consent to disclose this Agreement to the German tax authorities for purposes of obtaining such an exemption certificate.
7.9 Indirect Taxes. Amounts payable under this Agreement do not include any sales, use, excise, value added or other applicable taxes, tariffs or duties. If any taxing authority imposes a VAT, GST, sales, use, service, consumption, business or similar tax with respect to the work undertaken under this Agreement, then Xxxxxxx agrees to pay that amount if specified in a valid invoice or supply exemption documentation. For avoidance of doubt, Dyadic will not be entitled to pass on to Xxxxxxx, and Xxxxxxx will not be obligated to pay or bear, any tax that is based on Dyadic’s real, personal or intangible property (whether owned or leased), corporate structure, franchise, continuing business operations, income, gross receipts, capital stock, net worth or imposed with respect to Dyadic’s engagement of employees or independent contractors or that Dyadic incurs upon subcontracting any work hereunder, in whole or in part, to any Affiliate or Third Party. Dyadic is solely responsible, to the extent required by Applicable Law, for identifying, billing, and collecting the taxes payable by Xxxxxxx in all relevant federal, state, county, municipal and other taxing jurisdictions and for filing all required tax returns in a timely manner. To the extent that Dyadic does not provide Xxxxxxx a valid invoice (i.e., an invoice compliant with this Agreement and the rules and regulations of the jurisdiction of both Dyadic and Xxxxxxx, including separate identification of the tax where legally required), Dyadic shall be responsible for any penalty resulting directly from such noncompliance. The Parties will cooperate in good faith to minimize taxes to the extent legally permissible.
7.10 Records; Audits.
7.10.1 Research Plan Costs. During the Research Term and for a period of three full Calendar Years thereafter, Dyadic and its Affiliates shall keep complete and accurate financial records pertaining to FTE Costs and Out-Of-Pocket Expenses incurred by them in connection with the Dyadic Research Activities and FTEs and other out-of-pocket costs incurred by Dyadic in performing support activities hereunder in sufficient detail to permit Xxxxxxx to confirm the calculation of Reimbursable Research Costs and other reimbursed costs and the accuracy of Dyadic’s invoices delivered to Xxxxxxx pursuant to Section 7.2 (Research Plan Costs and Expenses) and otherwise hereunder. Xxxxxxx shall have the right, once annually and during normal business hours and on no less than 30 days’ prior written notice, to cause an independent, certified public accountant reasonably acceptable to Dyadic to audit such records to confirm the calculation of such Reimbursable Research Costs and other reimbursed costs and the accuracy of Dyadic’s invoices therefor for a period covering not more than the preceding three Calendar Years. The records for a given Calendar Year shall be subject to audit no more than one time.
7.10.2 Xxxxxxx Records. During the Term and for a period of three full Calendar Years thereafter, Xxxxxxx shall keep, and shall cause its Affiliates to keep, complete and accurate financial records pertaining to the Gross Mass of Xxxxxxx C1 Proteins by Xxxxxxx or its Affiliates to Third Parties in sufficient detail to permit Dyadic to confirm the accuracy of all Production-Based Milestone Payments due hereunder and Xxxxxxx’x reports with respect thereto, for at least three full Calendar Years following the end of the Calendar Year to which they pertain. Dyadic shall have the right, once every two years and during normal business hours and on no less than 30 days’ prior written notice, to cause an independent, certified public accountant that is an internationally recognized expert in the field of audit with offices in the Major Markets and reasonably acceptable to Xxxxxxx to audit such records (which records may be redacted to remove information that is not relevant to determining the Production-Based Milestone Payments) to confirm Production-Based Milestone Payments due hereunder, for a period covering not more than the preceding three full Calendar Years. The records for a given Calendar Year shall be subject to audit no more than one time.
7.10.3 Audit Procedures. The independent public accounting firm conducting an audit of a Party pursuant to Section 7.10.1 (Research Plan Costs) or Section 7.10.2 (Xxxxxxx Records) shall execute a reasonable confidentiality agreement with the audited Party prior to commencing any such inspection. Following completion of an inspection pursuant to Section 7.10.1 (Research Plan Costs) or Section 7.10.2 (Xxxxxxx Records), the independent public accounting firm shall, prior to distribution to the auditing Party, share its report with the audited Party. If the audited Party provides the independent public accounting firm with justifying remarks for inclusion in the report, the independent public accounting firm shall incorporate such remarks into its report prior to sharing the conclusions of such independent public accounting firm with the auditing Party. The final audit report shall be shared with both Parties at the same time and shall specify, as applicable: (a) whether any FTE Costs, FTEs, Out-Of-Pocket Expenses, or other out-of-pocket costs reported by Dyadic during the audited period were correct and, if incorrect, the difference between the reported amounts and the amounts actually incurred; or (b) whether any Production-Based Milestone Payments paid by Xxxxxxx during the audited period were correct and, if incorrect, the amount of any underpayment or overpayment. The audit report shall contain only such information as is reasonably necessary to provide the auditing Party with information regarding any actual or potential discrepancies. The auditing Party shall bear the costs and expenses of any inspection conducted under this Section 7.10 (Records; Audits) unless such inspection reveals, as applicable: (i) an over-reporting of FTE Costs, FTEs, Out-Of-Pocket Expenses, or other out-of-pocket costs by Dyadic in the audited period of more than 10% of the actual Reimbursable Research Costs incurred by Dyadic during such audited period, or (ii) an underpayment of Production-Based Milestone Payments payable by Xxxxxxx pursuant to Section 7.5 (Production-Based Milestones) in the audited period of more than 10% of the amount payable for the audited period, in which case ((i) or (ii)) the audited Party shall bear the costs and expenses of such inspection. If the audited Party disagrees with the findings of the audit report, the Parties will meet to attempt to mutually agree upon a resolution to the dispute. If such resolution cannot be reached, such disagreement shall be subject to the dispute resolution procedures set forth in ARTICLE 13 (Dispute Resolution).
7.10.4 Reconciliation.
(a) If an inspection of Dyadic’s records pursuant to Section 7.10.1 (Research Plan Costs) reveals an overpayment by Xxxxxxx of Reimbursable Research Costs, then Xxxxxxx shall invoice Dyadic for the excess of the amount paid over the amount actually due, and Xxxxxxx shall offset the invoiced amount against future payments due by Xxxxxxx to Dyadic if such future payments are due, otherwise Xxxxxxx shall invoice Dyadic for the excess of the amount actually due over the amount paid, and Dyadic shall pay such invoice within 90 days after receipt thereof. If an inspection of Dyadic’s records pursuant to Section 7.10.1 (Research Plan Costs) reveals an underpayment by Xxxxxxx of Reimbursable Research Costs, then Dyadic shall invoice Xxxxxxx for the excess of the amount actually due over the amount paid, and Xxxxxxx shall pay such invoice within 90 days after receipt thereof.
(b) If an inspection of Xxxxxxx’x records pursuant to Section 7.10.2 (Xxxxxxx Records) reveals an underpayment by Xxxxxxx, then Dyadic shall invoice Xxxxxxx for the amount of such underpayment, and Xxxxxxx shall pay such invoice within 90 days after receipt thereof. If an inspection of Xxxxxxx’x records pursuant to Section 7.10.2 (Xxxxxxx Records) reveals an overpayment by Xxxxxxx, then Xxxxxxx shall invoice Dyadic for the amount of the overpayment, and Xxxxxxx shall offset the invoiced amount against future payments due by Xxxxxxx to Dyadic if such future payments are due, otherwise Xxxxxxx shall invoice Dyadic for the excess of the amount actually due over the amount paid, and Dyadic shall pay such invoice within 90 days after receipt thereof.
7.10.5 Confidentiality. The receiving Party shall treat all information subject to review under this Section 7.10 (Records; Audits) in accordance with the confidentiality provisions of ARTICLE 9 (Confidentiality and Non-Disclosure).
7.11 No Other Compensation. Each Party hereby agrees that the terms of this Agreement fully define all consideration, compensation and benefits, monetary or otherwise, to be paid, granted or delivered by a Party to the other Party in connection with the transactions contemplated herein. Neither Party previously has paid or entered into any other commitment to pay, whether orally or in writing, any of the other Party’s employees, directly or indirectly, any consideration, compensation or benefits, monetary or otherwise, in connection with the transaction contemplated herein.
7.12 No Limitation. Nothing contained in this ARTICLE 7 (Payments and Records) shall in any way limit Xxxxxxx’x right to indemnification under this Agreement or to otherwise recover damages for breach of this Agreement.
7.13 Invoicing and Payments. Dyadic shall establish a payee profile with Xxxxxxx & Xxxxxxx Accounts Payable (J&J AP) and shall submit invoices electronically to J&J AP at xxx.xx.xxx.xxx. Invoices for undisputed amounts shall be due and payable [***] of Xxxxxxx’x receipt of a valid invoice, unless otherwise stated in the Agreement. All invoices will be paid via electronic funds transfer on the first or third Monday of the month. Payment run dates that fall on holidays will be paid on the next scheduled Business Day. Dyadic may contact J&J AP at 000-000-0000 to inquire about the status of submitted invoices. Invoices will not be processed without the following information: (a) invoice number, (b) invoice date, (c) amount of invoice, and (d) explanation of work completed.
7.14 Paying Agent. Xxxxxxx Research & Development, LLC, a New Jersey limited liability company having its principal place of business at 000 X.X. Xxxxx 000 (P.O. Box 300), Xxxxxxx, XX 00000, acting as paying agent for Xxxxxxx, may make certain payments due under this Agreement.
ARTICLE 8
INTELLECTUAL PROPERTY RIGHTS
8.1 Ownership of Intellectual Property Rights.
8.1.1 Dyadic Owned Rights. [***].
8.1.2 Xxxxxxx Owned Rights. [***].
8.1.3 Ownership of Jointly Owned Rights. [***].
8.1.4 United States Law. The determination of inventorship and whether Know-How (or Patent Rights Covering such Know-How) are developed or invented under this Agreement by a Party for the purpose of allocating proprietary rights (including Patent, copyright or other intellectual property rights) therein, shall, for purposes of this Agreement, be made in accordance with the United States patent law and other Applicable Law in the United States irrespective of where such development or invention occurs.
8.1.5 Covenants in Support of Assignment. Each Party will take (and cause its Designees to take) such further actions reasonably requested by the other Party to evidence the assignments of Patent Rights, Know-How, or Technology set forth in this Section 8.1 (Ownership of Intellectual Property Rights) and to assist the other Party in obtaining Patent Rights and other Intellectual Property protection for Inventions within the applicable ownership of such Patent Rights, Know-How, or Technology as provided for herein, as applicable, including executing further assignments, consents, releases, and other commercially reasonable documentation and providing good faith testimony by affidavit, declaration, in-person, or other proper means in support of any effort by the other Party to establish, perfect, defend, or enforce its rights in any such Patent Rights, Know-How, or Technology, as applicable, through prosecution of governmental filings, regulatory proceedings, litigation, and other means, including through the filing, prosecution, maintenance, and enforcement of such Patent Rights, Know-How, or Technology, as applicable. Without limitation, each Party will cooperate with the other Party if such other Party applies for U.S. or foreign patent protection for Inventions assigned by one Party to the other Party under this ARTICLE 8 (Intellectual Property Rights) and will obtain the cooperation of the individual inventors of any such Patent Rights, Know-How, or Technology, as applicable. If either Party is unable to assign any such Patent Rights, Know-How, or Technology, as applicable, to the other Party as set forth in this Section 8.1 (Ownership of Intellectual Property Rights), then each Party hereby grants and agrees to grant to the other Party a royalty-free, fully paid-up, worldwide, exclusive, perpetual, irrevocable license [***] under, as applicable, the Dyadic Owned Right or Xxxxxxx Owned Right, for any and all purposes.
8.2 Maintenance and Prosecution of Patent Rights.
8.2.1 By Dyadic. [***].
8.2.2 By Xxxxxxx. [***].
8.2.3 Cooperation. The Parties agree to cooperate fully in the preparation, filing, prosecution and maintenance of each Party’s interest in and to the Dyadic Owned Rights, the Xxxxxxx Owned Rights, and the Jointly Owned Rights in the Territory under this Agreement. Cooperation shall include:
(a) executing all papers and instruments, or requiring its employees or contractors to execute such papers and instruments, so as to (i) effectuate the ownership of intellectual property set forth in Section 8.1 (Ownership of Intellectual Property Rights); (ii) enable the other Party to apply for and to prosecute patent applications in the Territory; and (iii) obtain and maintain any patent term extensions, supplementary protection certificates and the like with respect to the Patent Rights included in the Dyadic Owned Rights, Xxxxxxx Owned Rights, and Jointly Owned Rights in the Territory, in each case ((i), (ii), and (iii)), to the extent provided for in this Agreement;
(b) consistent with this Agreement, assisting in any license, transfer or assignment registration processes with applicable governmental authorities that may be available in the Territory for the protection of a Party’s interests under this Agreement; and
(c) promptly informing the other Party of any matters coming to a Party’s attention that may materially affect the preparation, filing, prosecution or maintenance of any Patent Rights within the Dyadic Owned Rights, Xxxxxxx Owned Rights, and Jointly Owned Rights in the Territory.
8.2.4 Patent Term Extension and Supplementary Protection Certificate. [***].
8.2.5 Patent Listings. [***].
8.3.1 Notice of Infringement. Each Party shall promptly notify the other Party in writing of any alleged or threatened infringement of, as applicable, a Dyadic Owned Right, a Xxxxxxx Owned Right, or a Jointly Owned Right by a Third Party in the Territory of which such Party becomes aware (including alleged or threatened infringement based on the Development, Manufacture, Commercialization or Exploitation of, or an application to register or market, a product that would be competitive with a Xxxxxxx C1 Protein in the Territory (“Product Infringement”)).
(a) Enforcement of Dyadic Patent Rights. [***].
(b) Enforcement of Xxxxxxx Patent Rights. [***].
8.3.2 Enforcement of Jointly Owned Rights. [***].
8.3.3 Biosimilar Applications. [***].
8.3.4 Cooperation. The Parties agree to cooperate fully in any infringement action pursuant to this Section 8.3 (Enforcement of Patents). Where a Party brings such an action, the other Party shall, where necessary, furnish a power of attorney solely for such purpose or shall join in, or be named as a necessary party to, such action. Subject to the terms of this Agreement, the Party controlling any patent infringement litigation in accordance with this Section 8.3 (Enforcement of Patents) shall have the right to settle such claim; provided that neither Party shall have the right to settle any patent infringement litigation under this Section 8.3 (Enforcement of Patents) in a manner that imposes any costs or liability on, or involves any admission by, the other Party, without the express written consent of such other Party. The Party commencing the litigation shall keep the other Party reasonably informed of all material developments during the course of the proceedings.
8.3.5 Recovery. [***].
8.4 Infringement Claims by Third Parties. [***].
8.5 Invalidity, Unpatentability or Unenforceability Defenses or Actions.
8.5.1 Dyadic Patent Rights. [***].
8.5.2 Xxxxxxx Patent Rights. [***].
8.5.3 Jointly Owned Rights. [***].
8.5.4 Cooperation. Each Party shall assist and cooperate with the other Party as such other Party may reasonably request from time to time in connection with its activities set forth in this Section 8.5 (Invalidity, Unpatentability or Unenforceability Defenses or Actions), including by being joined as a party plaintiff in a claim, suit or proceeding, providing access to relevant documents and other evidence, and making its employees available during reasonable business hours. In connection with any such defense or claim or counterclaim, the controlling Party shall consider in good faith any comments from the other Party and shall keep the other Party reasonably informed of any material steps taken. In connection with the activities set forth in this Section 8.5 (Invalidity, Unpatentability or Unenforceability Defenses or Actions), each Party shall consult with the other as to the strategy for the defense of those Patent Rights as to which it has control over such actions. Subject to the terms of this Agreement, the Party controlling any such defense or claim or counterclaim shall have the right to settle such defense, claim or counterclaim; provided that neither Party shall have the right to settle any defense, claim or counterclaim in a manner that imposes any costs or liability on, or involves any admission by, the other Party, without the express written consent of such other Party.
8.5.5 Costs and Expenses. [***].
8.6 Third Party Licenses and Patents. [***].
8.7 Product Trademarks.
8.7.1 Ownership and Prosecution of Product Trademarks. Xxxxxxx shall own all rights, title and interests in and to the Product Trademarks in the Territory, and shall be responsible for the registration, prosecution and maintenance thereof. All costs and expenses of registering, prosecuting and maintaining the Product Trademarks shall be borne solely by Xxxxxxx.
8.7.2 Enforcement of Product Trademarks. Xxxxxxx shall have the sole right and responsibility for taking such action as Xxxxxxx deems necessary against a Third Party based on any alleged, threatened or actual infringement, dilution, misappropriation or other violation of, or unfair trade practices or any other like offense relating to, the Product Trademarks by a Third Party in the Territory. Xxxxxxx shall bear the costs and expenses relating to any enforcement action commenced pursuant to this Section 8.7.2 (Enforcement of Product Trademarks) and any settlements and judgments with respect thereto, and shall retain any damages or other amounts collected in connection therewith.
8.7.3 Third Party Claims. [***].
8.8 International Nonproprietary Name. As between the Parties, Xxxxxxx shall have the sole right and responsibility to select the International Nonproprietary Name or other name or identifier for any Xxxxxxx C1 Protein. Xxxxxxx shall have the sole right and responsibility to apply for submission to the World Health Organization for the International Nonproprietary Name, and submission to the United States Adopted Names Council for the United States Adopted Name.
8.9 Inventor’s Remuneration. Each Party shall be solely responsible for any remuneration that may be due such Party’s inventors under any applicable inventor remuneration laws.
8.10 Xxxxxxx Patent Rights. For clarity, Xxxxxxx shall have the sole right, but no obligation, to prosecute, maintain, enforce and defend any Patent Rights included in the Xxxxxxx Background Rights.
8.11 Common Interest. All information exchanged between the Parties regarding the prosecution, maintenance, enforcement and defense of Patent Rights under this ARTICLE 8 (Intellectual Property Rights) shall be deemed to be Confidential Information of the disclosing Party. In addition, the Parties acknowledge and agree that, with regard to such prosecution, maintenance, enforcement and defense, the interests of the Parties as collaborators, licensors or licensees are to, for their mutual benefit, obtain patent protection and plan patent defense against potential patentability or invalidity challenges or infringement activities by Third Parties, and, as such, are aligned and are legal in nature. The Parties agree and acknowledge that they have not waived, and nothing in this Agreement constitutes a waiver of, any legal privilege concerning Patent Rights under this ARTICLE 8 (Intellectual Property Rights), including privilege under the common interest doctrine and similar or related doctrines. Notwithstanding anything to the contrary in this Agreement, to the extent a Party has a good faith belief that any information required to be disclosed by such Party to the other Party under this ARTICLE 8 (Intellectual Property Rights) is protected by attorney-client privilege or any other applicable legal privilege or immunity, such Party shall not be required to disclose such information and the Parties shall in good faith cooperate to agree upon a procedure (which may include entering into a specific common interest agreement, disclosing such information on a “for counsel eyes only” basis or similar procedure) under which such information may be disclosed without waiving or breaching such privilege or immunity.
ARTICLE 9
CONFIDENTIALITY AND NON-DISCLOSURE
9.1 Confidentiality Obligations. At all times during the Term and for a period of 10 years following termination or expiration hereof in its entirety, each Party shall, and shall cause its officers, directors, employees and agents to, keep confidential and not publish or otherwise disclose to a Third Party and not use, directly or indirectly, for any purpose, any Confidential Information furnished or otherwise made known to it, directly or indirectly, by the other Party, except to the extent such disclosure or use is expressly permitted by the terms of this Agreement or otherwise reasonably necessary to exercise such Party’s rights or perform such Party’s obligations under this Agreement. The receiving Party shall use at least the same standard of care as it uses to protect proprietary or confidential information of its own, but no less than reasonable care, to ensure that its, and its Affiliates’, employees, agents, consultants, advisors, contractors and other representatives do not disclose or make any unauthorized use of the Confidential Information. The receiving Party will promptly notify the disclosing Party upon discovery of any unauthorized use or disclosure of the Confidential Information. All Data generated during the Research Term will be considered (a) during the Research Term, the Confidential Information of both Parties and (b) following Xxxxxxx’x exercise of the Research License Option in accordance with Section 4.2.1(b) (Exercise of Research License Option), the Confidential Information of Xxxxxxx. Additionally, (i) the terms of this Agreement and the Jointly Owned Rights will be considered the Confidential Information of both Parties, (ii) the identities of the Reserved Targets and the Xxxxxxx Proteins, the Xxxxxxx Background Rights and the Xxxxxxx Owned Rights will be considered the Confidential Information of Xxxxxxx, and (iii) the Licensed Intellectual Property (other than the Jointly Owned Rights) will be considered the Confidential Information of Dyadic.
9.2 Residual Knowledge. Notwithstanding the foregoing, the Parties acknowledge the practical difficulty of policing the use of information in the unaided memory of the receiving Party or its Affiliates and its and their officers, directors, employees and agents, and as such, each Party agrees that the receiving Party shall not be liable for the use by any of its or its Affiliates’ officers, directors, employees or agents of specific Confidential Information of the disclosing Party that is retained in the unaided memory of such officer, director, employee or agent; provided that (a) such officer, director, employee or agent is not aware that such Confidential Information is the confidential information of disclosing Party at the time of such use; (b) the foregoing is not intended to grant, and shall not be deemed to grant, the receiving Party, its Affiliates, or its officers, directors, employees and agents (i) a right to disclose the disclosing Party’s Confidential Information, or (ii) a license under any Patent Rights or other intellectual property right of the disclosing Party; and (c) such officer, director, employee or agent has not intentionally memorized such Confidential Information for use outside this Agreement.
9.3 Exceptions. Notwithstanding any provision to the contrary set forth in this Agreement, Confidential Information shall not include any information that the receiving Party can prove by competent evidence:
9.3.1 has been published by a Third Party or otherwise is or hereafter becomes part of the public domain by public use, publication, general knowledge or the like through no wrongful act, fault or negligence on the part of the receiving Party;
9.3.2 has been in the receiving Party’s possession prior to disclosure by the disclosing Party without any obligation of confidentiality with respect to such information; provided that the foregoing exception shall not apply with respect to Regulatory Documentation;
9.3.3 is subsequently received by the receiving Party from a Third Party without restriction and without breach of any agreement between such Third Party and the disclosing Party;
9.3.4 that is generally made available to Third Parties by the disclosing Party without restriction on disclosure; or
9.3.5 has been independently developed by or for the receiving Party without reference to, or use or disclosure of, the disclosing Party’s Confidential Information; provided that the foregoing exception shall not apply with respect to Regulatory Documentation.
Specific aspects or details of Confidential Information shall not be deemed to be within the public domain or in the possession of the receiving Party merely because such Confidential Information is embraced by more general information in the public domain or in the possession of the receiving Party. Further, any combination of Confidential Information shall not be considered in the public domain or in the possession of the receiving Party merely because individual elements of such Confidential Information are in the public domain or in the possession of the receiving Party unless the combination are in the public domain or in the possession of the receiving Party.
9.4 Permitted Disclosures. Each Party may disclose Confidential Information to the extent that such disclosure is:
9.4.1 in the reasonable opinion of the receiving Party’s legal counsel, required to be disclosed pursuant to law, regulation or a valid order of a court of competent jurisdiction or other supra-national, federal, national, regional, state, provincial and local governmental body of competent jurisdiction, (including by reason of filing with securities regulators, but subject to Section 9.6 (Public Announcements)); provided that the receiving Party shall first have given prompt written notice (and to the extent possible, at least five Business Days’ notice) to the disclosing Party and given the disclosing Party a reasonable opportunity to take whatever action it deems necessary to protect its Confidential Information (for example, quash such order or to obtain a protective order or confidential treatment requiring that the Confidential Information and documents that are the subject of such order be held in confidence by such court or governmental body or, if disclosed, be used only for the purposes for which the order was issued). In the event that no protective order or other remedy is obtained, or the disclosing Party waives compliance with the terms of this Agreement, the receiving Party shall furnish only that portion of Confidential Information that the receiving Party is advised by counsel is legally required to be disclosed;
9.4.2 made by or on behalf of the receiving Party to the Regulatory Authorities as required in connection with any filing, application or request for any IND or Regulatory Approval in accordance with the terms of this Agreement; provided that reasonable measures shall be taken to assure confidential treatment of such Confidential Information to the extent practicable and consistent with Applicable Law;
9.4.3 made by or on behalf of the receiving Party to a patent authority as may be necessary or reasonably useful for purposes of preparing, obtaining, defending or enforcing a Patent Right in accordance with the terms of this Agreement; provided that reasonable measures shall be taken to assure confidential treatment of such Confidential Information, to the extent such protection is available;
9.4.4 made to its or its Affiliates’ financial and legal advisors who have a need to know such disclosing Party’s Confidential Information and are either under professional codes of conduct giving rise to expectations of confidentiality and non-use or under written agreements of confidentiality and non-use, in each case, at least as restrictive as those set forth in this Agreement; provided that the receiving Party shall remain responsible for any failure by such financial and legal advisors to treat such Confidential Information as required under this ARTICLE 9 (Confidentiality and Non-Disclosure);
9.4.5 made by the receiving Party or its Affiliates to potential or actual investors or acquirers that is necessary in connection with their evaluation of such potential or actual investment or acquisition; provided that such Persons shall be subject to obligations of confidentiality and non-use with respect to such Confidential Information substantially similar to the obligations of confidentiality and non-use of the receiving Party pursuant to this ARTICLE 9 (Confidentiality and Non-Disclosure);
9.4.6 either Party may disclose of a copy of this Agreement to any tax authority (a) upon receipt of any legally enforceable information request by such tax authority, (b) in compliance with any legally enforceable filing requirement, or (c) in connection with a submitted transfer pricing analysis. In the event of any such disclosure, the disclosing Party will make reasonable efforts to ensure that the information is maintained in confidence by the applicable tax authority, including marking any disclosed document as confidential;
9.4.7 made by Xxxxxxx or its Affiliates to its or their advisors, consultants, clinicians, vendors, service providers, contractors, existing or prospective collaboration partners, licensees, [***] or other Third Parties as may be necessary or useful in connection with the Exploitation of the Xxxxxxx C1 Proteins or otherwise in connection with the performance of its obligations or exercise of its rights as contemplated by this Agreement; provided that such Persons shall be subject to obligations of confidentiality and non-use with respect to such Confidential Information substantially similar to the obligations of confidentiality and non-use of the receiving Party pursuant to this ARTICLE 9 (Confidentiality and Non-Disclosure) (with a duration of confidentiality and non-use obligations as appropriate that is no less than five years from the date of disclosure for advisors, consultants, clinicians, vendors, service providers and contractors); or
9.4.8 made by Dyadic or its Affiliates to its or their advisors, consultants, clinicians, vendors, service providers, contractors and the like to the extent necessary in assisting with Dyadic’s activities contemplated by this Agreement or to enjoy any right granted hereunder; provided that such Persons shall be subject to obligations of confidentiality and non-use with respect to such Confidential Information of Xxxxxxx substantially similar to the obligations of confidentiality and non-use of Dyadic pursuant to this ARTICLE 9 (Confidentiality and Non-Disclosure) (with a duration of confidentiality and non-use obligations as appropriate that is no less than five years from the date of disclosure).
9.5 Use of Name. Except as expressly provided herein, neither Party shall mention or otherwise use the name, logo or Trademark of the other Party or any of its Affiliates (or any abbreviation or adaptation thereof) in any publication, press release, marketing and promotional material, or other form of publicity, without the prior written approval of the other Party in each instance. The restrictions imposed by this Section 9.5 (Use of Name) shall not prohibit either Party from making any disclosure identifying the other Party that, in the opinion of the disclosing Party’s counsel, is required by Applicable Law; provided that such Party shall submit the proposed disclosure identifying the other Party in writing to the other Party as far in advance as reasonably practicable (and in no event less than three Business Days prior to the anticipated date of disclosure) so as to provide a reasonable opportunity to comment thereon.
9.6 Public Announcements. The Parties have agreed upon the content of a press release for Dyadic to release to the public regarding the execution of this Agreement, which shall be issued substantially in the form attached hereto as Schedule 9.6 (Press Release). Neither Party shall issue any other public announcement, press release or other public disclosure regarding this Agreement or its subject matter without the other Party’s prior written consent, except for any such disclosure that is, in the opinion of the disclosing Party’s counsel, required by Applicable Law or the rules of a stock exchange on which the securities of the disclosing Party are listed (or to which an application for listing has been submitted). In the event a Party is, in the opinion of its counsel, required by Applicable Law or the rules of a stock exchange on which its securities are listed (or to which an application for listing has been submitted) to make such a public disclosure, such Party shall submit the proposed disclosure in writing to the other Party as far in advance as reasonably practicable (and in no event less than three Business Days prior to the anticipated date of disclosure) so as to provide a reasonable opportunity to comment thereon. Notwithstanding the foregoing, Xxxxxxx, and its Affiliates shall have the right to publicly disclose research, development and commercial information (including with respect to regulatory matters) regarding the Xxxxxxx C1 Proteins; provided that (a) such disclosure shall be subject to the provisions of ARTICLE 9 (Confidentiality and Non-Disclosure) and Section 9.8 (Return of Confidential Information) with respect to Dyadic’s Confidential Information and (b) Xxxxxxx shall not use the name of Dyadic (or insignia, or any contraction, abbreviation or adaptation thereof) without Dyadic’s prior written permission.
9.7 Publications. Each Party recognizes that the publication of papers regarding results of, and other information regarding, activities under this Agreement, including oral presentations and abstracts, may be beneficial to both Parties so long as publications are subject to reasonable controls to protect Confidential Information. In particular, it is the intent of the Parties to maintain the confidentiality of any Confidential Information included in any invention disclosures or draft patent application until such patent application has been filed. Accordingly, each Party shall have the right to review and approve any paper proposed for publication by the other Party, including any oral presentation or abstract, that contains Data or pertains to results of Development activities, or other studies with respect to the Xxxxxxx C1 Proteins, or that includes Confidential Information of the other Party; provided that following the Research License Option Exercise Date, (a) Dyadic shall only have the right to publish papers regarding results of, and other information regarding, activities under this Agreement, including oral presentations and abstracts, with Xxxxxxx’x prior written consent, which consent shall be withheld or given in Xxxxxxx’x sole discretion, and (b) Dyadic shall only have the right to review and approve any paper proposed for publication by Xxxxxxx under this Agreement, including any oral presentation or abstract, if such proposed publication includes Confidential Information of Dyadic. Before any such paper or abstract is submitted for publication or an oral presentation is made, the publishing or presenting Party shall deliver a then-current copy of the paper, abstract or materials for oral presentation to the other Party at least 30 days prior to submitting the paper, abstract or materials to a publisher or making the presentation. The other Party shall review any such paper and give its comments to the publishing Party within 30 days of the delivery of such paper to the other Party. With respect to oral presentation materials and abstracts, the other Party shall make reasonable efforts to expedite review of such materials and abstracts, and shall return such items as soon as practicable to the publishing or presenting Party with appropriate comments, if any, but in no event later than 30 days from the date of delivery to the other Party. Failure to respond within such 30 days shall be deemed approval to publish or present. If approval is not given or deemed given, either Party may refer the matter to the JSC for resolution together with the reasons for withholding approval. Notwithstanding the foregoing, the publishing or presenting Party shall comply with the other Party’s request to delete references to the other Party’s Confidential Information in any paper, abstract or materials and shall withhold publication of any such paper, abstract or materials or any presentation of same for an additional 60 days in order to permit the Parties to obtain patent protection if either Party deems it necessary. Any publication shall include recognition of the contributions of the other Party according to standard practice for assigning scientific credit, either through authorship or acknowledgement, as may be appropriate.
9.8 Return of Confidential Information. Upon the effective date of the termination of this Agreement for any reason, a Party may request in writing, and the other Party shall either, with respect to Confidential Information (in the event of termination of this Agreement with respect to one or more countries, other jurisdictions or Terminated Territories but not in its entirety, solely to the extent relating specifically and exclusively to such countries, other territories or Terminated Territories) to which such other Party does not retain relevant rights under the surviving provisions of this Agreement: (a) as soon as reasonably practicable, destroy all copies of such Confidential Information in the possession of the other Party and confirm such destruction in writing to the requesting Party; or (b) as soon as reasonably practicable, deliver to the requesting Party, at the other Party’s expense, all copies of such Confidential Information in the possession of the other Party; provided that the other Party shall be permitted to retain one copy of such Confidential Information for the sole purpose of performing any continuing obligations hereunder, as required by Applicable Law, or for archival purposes. Notwithstanding the foregoing, the other Party also shall be permitted to retain such additional copies of or any computer records or files containing such Confidential Information that have been created solely by the other Party’s automatic archiving and back-up procedures, to the extent created and retained in a manner consistent with the other Party’s standard archiving and back-up procedures, but not for any other use or purpose.
9.9 Survival. All Confidential Information shall continue to be subject to the terms of this Agreement for the period set forth in Section 9.1 (Confidentiality Obligations).
ARTICLE 10
REPRESENTATIONS AND WARRANTIES
10.1 Mutual Representations and Warranties. Dyadic and Xxxxxxx each represents and warrants to the other, as of the Effective Date, as follows:
10.1.1 Organization. It is a duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization, and has all requisite power and authority, corporate or otherwise, to execute, deliver and perform this Agreement.
10.1.2 Authorization. The execution and delivery of this Agreement and the performance by it of the transactions contemplated hereby have been duly authorized by all necessary corporate action, and do not violate (a) such Party’s charter documents, bylaws or other organizational documents, (b) in any material respect, any agreement, instrument or contractual obligation to which such Party is bound, (c) any requirement of any Applicable Law, or (d) any order, writ, judgment, injunction, decree, determination or award of any court or governmental agency presently in effect applicable to such Party.
10.1.3 Binding Agreement. This Agreement is a legal, valid and binding obligation of such Party enforceable against it in accordance with its terms and conditions, subject to the effects of bankruptcy, insolvency or other laws of general application affecting the enforcement of creditor rights, judicial principles affecting the availability of specific performance and general principles of equity (whether enforceability is considered a proceeding at law or equity).
10.1.4 No Inconsistent Obligation. It is not under any obligation, contractual or otherwise, to any Person that conflicts with or is inconsistent in any material respect with the terms of this Agreement, or that would impede the diligent and complete fulfillment of its obligations hereunder.
10.2 Additional Representations, Warranties of Dyadic. [***]:
10.2.1 [***].
10.2.2 [***].
10.2.3 [***].
10.2.4 [***].
10.2.5 [***].
10.2.6 [***].
10.2.7 [***].
10.2.8 [***].
10.2.9 [***].
10.2.10 [***].
10.2.11 [***].
10.2.12 [***].
10.2.13 [***]:
(a) A “Debarred Individual” is an individual who has been debarred by the FDA pursuant to 21 U.S.C. §335a (a) or (b) from providing services in any capacity to a person that has an approved or pending drug or biological product application.
(b) A “Debarred Entity” is a corporation, partnership or association that has been debarred by the FDA pursuant to 21 U.S.C. §335a (a) or (b) from submitting or assisting in the submission of any abbreviated drug application, or a subsidiary or affiliate of a Debarred Entity.
(c) An “Excluded Individual” or “Excluded Entity” is (i) an individual or entity, as applicable, who has been excluded, debarred, suspended or is otherwise ineligible to participate in federal health care programs such as Medicare or Medicaid by the Office of the Inspector General (OIG/HHS) of the U.S. Department of Health and Human Services, or (ii) is an individual or entity, as applicable, who has been excluded, debarred, suspended or is otherwise ineligible to participate in federal procurement and non-procurement programs, including those produced by the U.S. General Services Administration (GSA).
(d) A “Convicted Individual” or “Convicted Entity” is an individual or entity, as applicable, who has been convicted of a criminal offense that falls within the ambit of 21 U.S.C. §335a (a) or 42 U.S.C. §1320a - 7(a), but has not yet been excluded, debarred, suspended or otherwise declared ineligible.
10.2.14 [***].
10.2.15 [***].
10.2.16 [***].
10.2.17 [***].
10.3 Covenants. Dyadic further covenants and agrees that:
10.3.1 [***].
10.3.2 [***].
10.3.3 [***].
10.3.4 [***].
10.3.5 [***].
10.3.6 [***].
10.3.7 [***].
10.3.8 [***].
10.3.9 [***].
10.3.10 [***].
10.3.11 [***].
10.3.12 [***].
10.3.13 [***].
10.3.14 [***].
10.3.15 [***].
10.4 DISCLAIMER OF WARRANTIES. EXCEPT FOR THE EXPRESS WARRANTIES SET FORTH HEREIN, NEITHER PARTY MAKES ANY REPRESENTATIONS OR GRANTS ANY WARRANTIES, EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE, AND EACH PARTY SPECIFICALLY DISCLAIMS ANY OTHER WARRANTIES, WHETHER WRITTEN OR ORAL, OR EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF QUALITY, MERCHANTABILITY OR FITNESS FOR A PARTICULAR USE OR PURPOSE.
ARTICLE 11
INDEM[***]NITY
11.1 Indemnification of Dyadic.
.
11.2 Indemnification of Xxxxxxx. [***]
.
11.3 Notice of Claim. All indemnification claims in respect of a Party, its Affiliates or their respective directors, officers, employees and agents shall be made solely by such Party to this Agreement (the “Indemnified Party”). The Indemnified Party shall give the indemnifying Party prompt written notice (an “Indemnification Claim Notice”) of any Losses or discovery of fact upon which such Indemnified Party intends to base a request for indemnification under this ARTICLE 11 (Indemnity), but in no event shall the indemnifying Party be liable for any Losses that result from any delay in providing such notice. Each Indemnification Claim Notice must contain a description of the claim and the nature and amount of the Loss (to the extent that the nature and amount of such Loss is known at such time). The Indemnified Party shall furnish promptly to the indemnifying Party copies of all papers and official documents received in respect of any Losses and Third Party Claims.
11.4 Control of Defense.
11.4.1 In General. [***].
11.4.2 Right to Participate in Defense. [***].
11.4.3 Settlement. [***].
11.4.4 Cooperation. Regardless of whether the indemnifying Party chooses to defend or prosecute any Third Party Claim, the Indemnified Party shall, and shall cause each indemnitee to, cooperate in the defense or prosecution thereof and shall furnish such records, information and testimony, provide such witnesses and attend such conferences, discovery proceedings, hearings, trials and appeals as may be reasonably requested in connection therewith. Such cooperation shall include access during normal business hours afforded to the indemnifying Party to, and reasonable retention by the Indemnified Party of, records and information that are reasonably relevant to such Third Party Claim, and making Indemnified Parties and other employees and agents available on a mutually convenient basis to provide additional information and explanation of any materials provided hereunder, and the indemnifying Party shall reimburse the Indemnified Party for all its reasonable out-of-pocket expenses in connection therewith.
11.4.5 Expenses. [***].
11.5 Special, Indirect, and Other Losses. EXCEPT (A) FOR WILLFUL MISCONDUCT OR GROSS NEGLIGENCE, (B) FOR A PARTY’S BREACH OF ITS OBLIGATIONS UNDER ARTICLE 9 (CONFIDENTIALITY AND NON-DISCLOSURE), (C) AS PROVIDED UNDER SECTION 14.9 (EQUITABLE RELIEF) AND (D) TO THE EXTENT ANY SUCH DAMAGES ARE REQUIRED TO BE PAID TO A THIRD PARTY AS PART OF A CLAIM FOR WHICH A PARTY PROVIDES INDEMNIFICATION UNDER THIS ARTICLE 11 (INDEMNITY), NEITHER PARTY NOR ANY OF ITS AFFILIATES SHALL BE LIABLE FOR INDIRECT, INCIDENTAL, SPECIAL, EXEMPLARY, PUNITIVE OR CONSEQUENTIAL DAMAGES, INCLUDING LOSS OF PROFITS OR BUSINESS INTERRUPTION, HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY, WHETHER IN CONTRACT, TORT, NEGLIGENCE, BREACH OF STATUTORY DUTY OR OTHERWISE IN CONNECTION WITH OR ARISING IN ANY WAY OUT OF THE TERMS OF THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGE.
11.6 Insurance. During the Term and for a period of not less than three years thereafter, each Party will maintain, at its sole cost, reasonable insurance against liability and other risks associated with its activities contemplated by this Agreement, which insurance will be consistent with the normal and customary practices of companies of similar size, nature and scope. Upon written request, each Party will provide evidence of insurance in the form of a certificate of insurance to the requesting Party. Each Party shall provide the other Party with 30 days advance written notice in the event of any insurance cancellation.
ARTICLE 12
TERM AND TERMINATION
12.1 Term. This Agreement shall commence on the Effective Date and, unless earlier terminated in accordance herewith, shall continue in force and effect until: (a) in the event that the Research License Option is not exercised by Xxxxxxx during the Initial Agreement Term, the conclusion of the Initial Agreement Term, or (b) in the event that the Research License Option is exercised by Xxxxxxx during the Initial Agreement Term, then the expiration of the last to expire Program Payment Term (the “Term”).
12.2 Termination for Material Breach. If a Party (the “Non-Breaching Party”) believes that the other Party (the “Breaching Party”) has materially breached this Agreement, then the Non-Breaching Party may deliver notice of such material breach to the Breaching Party (a “Default Notice”). If the Breaching Party does not dispute that it has committed a material breach of this Agreement, then if the Breaching Party fails to cure such material breach, or fails to take steps as would be considered reasonable to effectively cure such material breach, within 90 days after receipt of the Default Notice, or if such compliance cannot be fully achieved within such 90-day period and the Breaching Party has failed to commence compliance or has failed to use diligent efforts to achieve full compliance as soon thereafter as is reasonably possible, the Non-Breaching Party may terminate this Agreement upon written notice to the Breaching Party. Except as expressly set forth herein, the termination right provided for in this Section 12.2 (Termination for Material Breach) is not a condition precedent to the initiation of a claim of breach under this Agreement or any claim under Applicable Laws or in equity.
12.3 Termination Right by Xxxxxxx For Convenience. Xxxxxxx may terminate this Agreement in its entirety, or on a country-by-country or other jurisdiction-by-other jurisdiction basis, for any or no reason, upon 90 days’ prior written notice to Dyadic.
12.4 Termination for Insolvency. In the event that either Party (a) files for protection under bankruptcy or insolvency laws, (b) makes an assignment for the benefit of creditors, (c) appoints or suffers appointment of a receiver or trustee over substantially all of its property that is not discharged within 90 days after such filing, (d) proposes a written agreement of composition or extension of its debts, (e) proposes or is a party to any dissolution or liquidation, (f) files a petition under any bankruptcy or insolvency act or has any such petition filed against it that is not discharged within 60 days of the filing thereof, or (g) admits in writing its inability generally to meet its obligations as they fall due in the general course, then the other Party may terminate this Agreement in its entirety effective immediately upon written notice to such Party.
12.5 Rights in Bankruptcy.
12.5.1 Applicability of 11 U.S.C. § 365(n). All rights and licenses (collectively, the “Intellectual Property”) granted under or pursuant to this Agreement, including all rights and licenses to use improvements or enhancements developed during the Term, are intended to be, and shall otherwise be deemed to be, for purposes of Section 365(n) of the United States Bankruptcy Code (the “Bankruptcy Code”) or any analogous provisions in any other country or jurisdiction, licenses of rights to “intellectual property” as defined under Section 101(35A) of the Bankruptcy Code. The Parties agree that the licensee of such Intellectual Property under this Agreement shall retain and may fully exercise all of its rights and elections under the Bankruptcy Code, including Section 365(n) of the Bankruptcy Code, or any analogous provisions in any other country or jurisdiction. All of the rights granted to either Party under this Agreement shall be deemed to exist immediately before the occurrence of any bankruptcy case in which the other Party is the debtor. Without limiting the generality of the foregoing, Dyadic and Xxxxxxx intend and agree that any sale of Dyadic’s assets under Section 363 of the Bankruptcy Code shall be subject to Xxxxxxx’x rights under Section 365(n), that Xxxxxxx cannot be compelled to accept a money satisfaction of its interests in the intellectual property licensed pursuant to this Agreement, and that any such sale therefore may not be made to a purchaser “free and clear” of Xxxxxxx’x rights under this Agreement and Section 365(n) without the express, contemporaneous consent of Xxxxxxx. Dyadic will, during the term of this Agreement, create and maintain current copies or, if not amenable to copying, detailed descriptions or other appropriate embodiments, to the extent feasible, of all such intellectual property.
12.5.2 Rights of non-Debtor Party in Bankruptcy. If a bankruptcy proceeding is commenced by or against either Party under the Bankruptcy Code or any analogous provisions in any other country or jurisdiction, the non-debtor Party shall be entitled to a complete duplicate of (or complete access to, as appropriate) any Intellectual Property and all embodiments of such Intellectual Property, which, if not already in the non-debtor Party’s possession, shall be delivered to the non-debtor Party within five Business Days of such request; provided that the debtor Party shall be excused from its obligation to deliver the Intellectual Property to the extent the debtor Party continues to perform all of its obligations under this Agreement and the Agreement has not been rejected pursuant to the Bankruptcy Code or any analogous provision in any other country or jurisdiction. Dyadic (in any capacity, including debtor-in-possession) and its successors and assigns (including a trustee) shall not interfere with Xxxxxxx’x rights under this Agreement, or any agreement supplemental hereto, to such intellectual property (including such embodiments), including any right to obtain such intellectual property (or such embodiments) from another entity, to the extent provided in Section 365(n) of the Bankruptcy Code.
12.5.3 Right of Access. All rights, powers and remedies of Xxxxxxx provided herein are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity (including the Bankruptcy Code) in the event of the commencement of a case under the Bankruptcy Code with respect to Dyadic. The Parties agree that they intend the following rights to extend to the maximum extent permitted by law, and to be enforceable under Bankruptcy Code Section 365(n):
(a) the right of access to any intellectual property (including all embodiments thereof) of Dyadic, or any Third Party with whom Dyadic contracts to perform an obligation of Dyadic under this Agreement, and, in the case of the Third Party, which is necessary for the practice of the licensed intellectual property rights or the manufacture, use, sale, import or export of Xxxxxxx C1 Proteins; and
(b) the right to contract directly with any Third Party to perform contracted work.
12.6 Modification in Lieu of Termination. [***]:
12.6.1 [***];
12.6.2 In the event that Xxxxxxx delivers notice to Dyadic of its exercise of the right to conduct activities allocated to Dyadic under the Research Plan prior to the Research License Option Exercise Date, Dyadic hereby grants, and shall cause its Affiliates to grant, to Xxxxxxx a license, with the right to grant sublicenses to its Affiliates, under the Licensed Intellectual Property to conduct activities allocated to Xxxxxxx under the Research Plan;
12.6.3 Xxxxxxx shall have the right, at Xxxxxxx’x sole election, to disband the JSC and terminate the activities of the JSC and thereafter undertake all activities assigned by this Agreement to the JSC solely and exclusively by itself;
12.6.4 if Xxxxxxx has such right to terminate prior to the Research License Option Exercise Date, the Research License Option Exercise Fee shall be reduced by [***];
12.6.5 the amount of any milestone payment payable by Xxxxxxx to Dyadic under Section 7.4 (Development and Commercial Milestones) and Section 7.5 (Production-Based Milestones) for any milestone event achieved after Xxxxxxx has such right to terminate shall be reduced by [***] of the applicable amount set forth in Section 7.4 (Development and Commercial Milestones) or Section 7.5 (Production-Based Milestones), as applicable; and
12.6.6 all other provisions of this Agreement shall remain in full force and effect without change.
12.7 Effects of Termination.
12.7.1 Termination in Entirety. In the event of a termination of this Agreement in its entirety for any reason (other than expiration of the Term following Xxxxxxx’x exercise of the Research License Option), including in the event that the Term expires because Xxxxxxx does not exercise the Research License Option prior to the expiration of the Initial Agreement Term:
(a) all rights, licenses and options granted by Dyadic hereunder shall immediately terminate other than those pursuant to Section 5.5 (Unblocking Licenses);
(b) all rights and licenses granted by Xxxxxxx hereunder shall immediately terminate other than those pursuant to Section 5.5 (Unblocking Licenses);
(c) except as set forth in Section 12.10 (Accrued Rights; Surviving Obligations), as of the effective date of termination, all rights and obligations of the Parties under this Agreement will terminate;
(d) promptly after termination of this Agreement in its entirety, each receiving Party will return to the disclosing Party or destroy, at the disclosing Party’s election, all Confidential Information of the disclosing Party that is in the possession or control of the receiving Party in accordance with Section 9.8 (Return of Confidential Information) provided, however, that nothing in this Agreement will prevent a Party retaining any records as required by Applicable Law; and
(e) each Party will destroy any cell lines or other Materials owned or supplied by the other Party in connection with this Agreement or that were used to produce Xxxxxxx Proteins and, subject to Section 9.2 (Residual Knowledge), each Party will covenant not use or gain any benefit from any Know-How or Technology relating the other Party’s Materials or, in the case of Dyadic, relating exclusively to a Xxxxxxx C1 Protein.
12.7.2 Termination During Research Term. In the event of a termination of this Agreement in its entirety during the Research Term for any reason other than by Xxxxxxx pursuant to Section 12.2 (Termination For Material Breach), Xxxxxxx shall reimburse Dyadic for any non-cancellable Reimbursable Research Costs incurred by Dyadic prior to delivery of applicable written notice of termination. Upon receipt or delivery (as applicable) of notice of termination of this Agreement, Dyadic will promptly terminate any outstanding commitments and avoid incurring any further costs under the Research Plan. Dyadic shall provide to Xxxxxxx a reconciliation of such Reimbursable Research Costs incurred, together with the applicable report and documentation required under Section 7.2 (Research Plan Costs and Expenses) and an invoice for the amount due, within [***] of notice of termination and Xxxxxxx shall pay such undisputed amounts within [***] of receipt of a valid invoice. If any reconciliation of Reimbursable Research Costs reveals an overpayment by Xxxxxxx, then Dyadic shall pay such overpayment to Xxxxxxx within [***] following receipt of an invoice therefore from Xxxxxxx.
12.7.3 Termination of Terminated Territory. In the event of a termination of this Agreement with respect to a country or other jurisdiction by Xxxxxxx pursuant to Section 12.3 (Termination Right by Xxxxxxx For Convenience) (but not in the case of any termination of this Agreement in its entirety), all rights and licenses granted by Dyadic hereunder (a) shall automatically be deemed to be amended to exclude, if applicable and subject to Section 12.7.3(b) (Termination of Terminated Territory), the right to Exploit the terminated Xxxxxxx C1 Proteins in such country or other jurisdiction or Terminated Territory, as applicable, and (b) shall otherwise survive and continue in effect in such country or other jurisdiction or Terminated Territory, as applicable, solely for the purpose of furthering any Exploitation of the Xxxxxxx C1 Proteins in the Territory excluding the Terminated Territory.
12.8 Remedies. Except as otherwise expressly provided herein, termination of this Agreement (either in its entirety or with respect to one or more country(ies) or other jurisdiction(s)) in accordance with the provisions hereof shall not limit remedies that may otherwise be available in law or equity.
12.9 Effects of Expiration. If the Term is extended beyond the Initial Agreement Term because Xxxxxxx exercised the Research License Option, then, upon expiration of the Term, the licenses granted to Xxxxxxx under ARTICLE 5 (License Grants) will become fully paid up, perpetual and irrevocable, [***], with respect to the Exploitation of Xxxxxxx C1 Proteins that were the subject of Programs for which Xxxxxxx exercised a Commercial License Option during the Term.
12.10 Accrued Rights; Surviving Obligations. Termination or expiration of this Agreement (either in its entirety or with respect to one or more country(ies) or other jurisdiction(s)) for any reason shall be without prejudice to any rights that shall have accrued to the benefit of a Party prior to such termination or expiration. Such termination or expiration shall not relieve a Party from obligations that are expressly indicated to survive the termination or expiration of this Agreement. Without limiting the foregoing, ARTICLE 1 (Definitions), Section 2.5 (Records) (for the time period set forth therein), Section 2.7.3 (Return or Destruction of Materials), Section 7.5.6 (Expiration of Program Payment Term), Section 7.10.1 (Research Plan Costs) (for the time period set forth therein), Section 7.10.2 (Xxxxxxx Records) (for the time period set forth therein), Section 7.10.5 (Confidentiality), Section 8.1 (Ownership of Intellectual Property Rights), ARTICLE 9 (Confidentiality and Non-Disclosure) (for the time period set forth therein), Section 10.3.5, ARTICLE 11 (Indemnity), Section 12.1 (Term), Section 12.7 (Effects of Termination), Section 12.8 (Remedies), Section 12.9 (Effects of Expiration), this Section 12.10 (Accrued Rights; Surviving Obligations), ARTICLE 13 (Dispute Resolution), and ARTICLE 14 (Miscellaneous) of this Agreement shall survive the termination or expiration of this Agreement for any reason. If this Agreement is terminated with respect to a country, other jurisdiction or a Terminated Territory but not in its entirety, then following such termination, the foregoing provisions of this Agreement shall remain in effect with respect to such country, other jurisdiction or Terminated Territory, as applicable, (to the extent they would survive and apply in the event the Agreement expires or is terminated in its entirety) and all provisions not surviving in accordance with the foregoing shall terminate upon termination of this Agreement with respect to such country, other jurisdiction or Terminated Territory and be of no further force and effect (and, for purposes of clarity, all provisions of this Agreement shall remain in effect with respect to all countries in the Territory other than such country, other jurisdiction or Terminated Territory).
ARTICLE 13 DISPUTE RESOLUTION
13.1 Resolution of Disputes. The Parties shall negotiate in good faith and use reasonable efforts to settle any dispute, controversy or claim arising from or related to this Agreement or the breach thereof (each, a “Dispute”). If the Parties are unable to resolve a Dispute within 30 days despite using reasonable efforts to do so, either Party may, by written notice to the other, have the dispute referred to their respective senior management designated below or their respective successors, for attempted resolution by negotiation in good faith. The attempted resolution will take place no later than 30 days following receipt of such notice. The designated management (each designated representative, an “Executive Officer”) are as follows:
For Dyadic: [***]
For Xxxxxxx: [***]
If the Parties are unable to resolve the Dispute within 30 days following the day on which one Party provides written notice of the Dispute to the other in accordance with this Section 13.1 (Resolution of Disputes), and a Party wishes to pursue the matter, each such Dispute will be finally resolved by mediation followed by binding arbitration as set forth below in this ARTICLE 13 (Dispute Resolution).
13.2 Mediation.
13.2.1 CPR Mediation Procedure. [***].
13.2.2 Conduct of Mediation. [***].
13.2.3 Extension of Limitations. The Parties agree that any period of limitations that would otherwise expire between the initiation of mediation and its conclusion is hereby extended until 20 days after the conclusion of the mediation.
13.3 Arbitration.
13.3.1 CPR Rules. [***].
13.3.2 Qualifications of Arbitrators. [***].
13.3.3 Number of Arbitrators. [***].
13.3.4 Interview of Arbitrators. [***].
13.3.5 Timing. [***].
13.3.6 Evidence. [***].
13.3.7 Hearing. [***].
13.3.8 Decision of Arbitrators. [***]
13.3.9 Opinion; Costs. [***].
13.3.10 Enforcement. The award may be entered and enforced in any court of competent jurisdiction. If a court is called upon to enforce an award in a court proceeding, the Parties consent to the court’s requiring the Party resisting enforcement to pay the reasonable attorneys’ fees and costs incurred in that proceeding by the Party seeking enforcement.
13.3.11 Interim Relief. Any Party may seek emergency, interim, or provisional relief before the appointment of the arbitrator(s) from any court of competent jurisdiction, without waiver of the agreements to mediate and arbitrate. After appointment of the arbitrator(s), any request for emergency, interim, or provisional relief shall either be addressed to the arbitrator(s), which shall have the power to enter an interim award granting relief using the standards provided by Applicable Law, or to a court, but only with the permission of the arbitrator(s). Any interim award of the arbitrator(s) may be enforced in any court of competent jurisdiction.
13.3.12 Waiver of Jury Trial. [***].
ARTICLE 14
MISCELLANEOUS
14.1 Force Majeure. Neither Party shall be held liable or responsible to the other Party or be deemed to have defaulted under or breached this Agreement for failure or delay in fulfilling or performing any term of this Agreement when such failure or delay is caused by or results from events beyond the reasonable control of the non-performing Party, including fires, floods, earthquakes, hurricanes, embargoes, shortages, epidemics, quarantines, war, acts of war (whether war be declared or not), terrorist acts, insurrections, riots, civil commotion, strikes, lockouts or other labor disturbances (whether involving the workforce of the non-performing Party or of any other Person), acts of God or acts, omissions or delays in acting by any governmental authority (except to the extent such delay results from the breach by the non-performing Party or any of its Affiliates of any term or condition of this Agreement). The non-performing Party shall notify the other Party of such force majeure within 30 days after such occurrence by giving written notice to the other Party stating the nature of the event, its anticipated duration and any action being taken to avoid or minimize its effect. The suspension of performance by the non-performing Party shall be of no greater scope and no longer duration than is necessary and the non-performing Party shall use commercially reasonable efforts to remedy its inability to perform.
14.2 Export Control. This Agreement is made subject to any restrictions concerning the export of products or technical information from the United States or other countries that may be imposed on the Parties from time to time. Each Party agrees that it shall not export, directly or indirectly, any technical information acquired from the other Party under this Agreement or any products using such technical information to a location or in a manner that at the time of export requires an export license or other governmental approval, without first obtaining the written consent to do so from the appropriate agency or other governmental entity in accordance with Applicable Law.
14.3 Assignment. Without the prior written consent of the other Party, such consent not to be unreasonably withheld, conditioned or delayed, neither Party shall sell, transfer, assign, delegate, pledge or otherwise dispose of, whether voluntarily, involuntarily, by operation of law or otherwise, this Agreement or any of its rights or duties hereunder; provided that (a) at any time during the Term, Xxxxxxx may make such an assignment without Dyadic’s consent to its Affiliate or to a successor, whether in a merger, sale of stock, sale of assets, or any other transaction, of the business to which this Agreement relates and (b) at any time during the Term that is 90 days or more following the Research License Option Exercise Date and completion of the Cell Line Technology Transfer, Dyadic may make such an assignment without Xxxxxxx’x consent to its Affiliate or to a successor, whether in a merger, sale of stock, sale of assets, or any other transaction, of the business to which this Agreement relates. With respect to an assignment to an Affiliate, the assigning Party shall remain responsible for the performance by such Affiliate of the assigning Party’s rights and obligations hereunder. Any attempted assignment or delegation in violation of this Section 14.3 (Assignment) shall be void and of no effect. All validly assigned and delegated rights and obligations of the Parties hereunder shall be binding upon and inure to the benefit of and be enforceable by and against the successors and permitted assigns of Dyadic or Xxxxxxx, as the case may be. The permitted assignee or transferee shall assume all obligations of its assignor or transferor under this Agreement. Without limiting the foregoing, the grant of rights set forth in this Agreement shall be binding upon any successor or permitted assignee of Dyadic, and the obligations of Xxxxxxx, including the payment obligations, shall run in favor of any such successor or permitted assignee of Dyadic’s benefits under this Agreement.
14.4 Severability. If any provision of this Agreement is held to be illegal, invalid or unenforceable under any present or future law, and if the rights or obligations of either Party under this Agreement shall not be materially and adversely affected thereby, (a) such provision shall be fully severable, (b) this Agreement shall be construed and enforced as if such illegal, invalid or unenforceable provision had never comprised a part hereof, (c) the remaining provisions of this Agreement shall remain in full force and effect and shall not be affected by the illegal, invalid or unenforceable provision or by its severance herefrom, and (d) in lieu of such illegal, invalid or unenforceable provision, there shall be added automatically as a part of this Agreement a legal, valid and enforceable provision as similar in terms to such illegal, invalid or unenforceable provision as may be possible and reasonably acceptable to the Parties. To the fullest extent permitted by Applicable Law, each Party hereby waives any provision of law that would render any provision hereof illegal, invalid or unenforceable in any respect.
14.5 Governing Law; Service.
14.5.1 Governing Law. This Agreement and any disputes, claims, or actions related thereto shall be governed by and construed in accordance with the laws of the [***], USA, without regard to any conflicts of law provisions thereof that might otherwise refer construction or interpretation of this Agreement to the substantive law of another jurisdiction; provided that all questions concerning (a) inventorship of Patent Rights under this Agreement shall be determined in accordance with Section 8.1.4 (United States Law) and (b) the construction or effect of Patent Rights shall be determined in accordance with the laws of the country or other jurisdiction in which the particular Patent Right has been filed or granted, as the case may be.
14.5.2 Service. Each Party further agrees that service of any process, summons, notice or document by registered mail to its address set forth in Section 14.6.2 (Address for Notice) shall be effective service of process for any action, suit or proceeding brought against it under this Agreement in any court.
14.6 Notices.
14.6.1 Notice Requirements. Any notice, request, demand, waiver, consent, approval or other communication permitted or required under this Agreement shall be in writing, shall refer specifically to this Agreement and shall be deemed given only if (a) delivered by hand, (b) sent by facsimile transmission (with transmission confirmed), or (c) by internationally recognized overnight delivery service that maintains records of delivery, addressed to the Parties at their respective addresses specified in Section 14.6.2 (Address for Notice) or to such other address as the Party to whom notice is to be given may have provided to the other Party in accordance with this Section 14.6.1 (Notice Requirements). Such notice shall be deemed to have been given as of the date delivered by hand or transmitted by facsimile (with transmission confirmed) or on the second Business Day (at the place of delivery) after deposit with an internationally recognized overnight delivery service. Any notice delivered by facsimile shall be confirmed by a hard copy delivered as soon as practicable thereafter. This Section 14.6.1 (Notice Requirements) is not intended to govern the day-to-day business communications necessary between the Parties in performing their obligations under the terms of this Agreement.
14.6.2 Address for Notice.
If to Janssen, to:
Xxxxxxx Biotech, Inc.
000/000 Xxxxxxxxx Xxxxx
Xxxxxxx, XX 00000
Attention: President
and
Xxxxxxx & Xxxxxxx
Law Department
Xxx Xxxxxxx & Xxxxxxx Xxxxx
Xxx Xxxxxxxxx, Xxx Xxxxxx 00000
Attention: Chief Intellectual Property Counsel
fax: [***]
with a copy (which shall not constitute notice) to:
Ropes & Xxxx LLP
000 Xxxxxxxx Xxxxxx, Xxxxxxxxxx Xxxxx
Xxxxxx, XX 00000
Attention: [***]
email: [***]
If to Dyadic, to:
Dyadic International, Inc.
000 Xxxxxxxxxxxx Xxxxxx Xxxxx
Xxxxx # 000
Xxxxxxx, Xxxxxxx 00000
Attention: [***]
fax: [***]
email: [***]
with a copy (which shall not constitute notice) to:
Xxxxxx Xxxxxx & Xxxxxxx LLP
00 Xxx Xxxx
Xxx Xxxx, XX 00000
Attention: [***]
email: [***]
14.7 Entire Agreement; Amendments. This Agreement, together with the Schedules attached hereto, sets forth and constitutes the entire agreement and understanding between the Parties with respect to the subject matter hereof and all prior agreements, understandings, promises and representations, whether written or oral, with respect thereto are superseded hereby (including the CDA). Each Party confirms that it is not relying on any representations or warranties of the other Party except as specifically set forth in this Agreement. No amendment, modification, release or discharge shall be binding upon the Parties unless in writing and duly executed by authorized representatives of both Parties.
14.8 English Language. This Agreement shall be written and executed in, and all other communications under or in connection with this Agreement shall be in, the English language. Any translation into any other language shall not be an official version thereof, and in the event of any conflict in interpretation between the English version and such translation, the English version shall control.
14.9 Equitable Relief. Each Party acknowledges and agrees that the restrictions set forth in ARTICLE 8 (Intellectual Property Rights) and ARTICLE 9 (Confidentiality and Non-Disclosure) are reasonable and necessary to protect the legitimate interests of the other Party and that such other Party would not have entered into this Agreement in the absence of such restrictions, and that any breach or threatened breach of any provision of such Section or Articles may result in irreparable injury to such other Party for which there shall be no adequate remedy at law. In the event of a breach or threatened breach of any provision of such Section or Articles, the non-breaching Party shall be authorized and entitled to seek from any court of competent jurisdiction injunctive relief, whether preliminary or permanent, specific performance and an equitable accounting of all earnings, profits and other benefits arising from such breach, which rights shall be cumulative and in addition to any other rights or remedies to which such non-breaching Party may be entitled in law or equity. Both Parties agree to waive any requirement that the other Party (a) post a bond or other security as a condition for obtaining any such relief, and (b) show irreparable harm, balancing of xxxxx, consideration of the public interest or inadequacy of monetary damages as a remedy. Nothing in this Section 14.9 (Equitable Relief) is intended, or should be construed, to limit either Party’s right to equitable relief or any other remedy for a breach of any other provision of this Agreement.
14.10 Waiver and Non-Exclusion of Remedies. Any term or condition of this Agreement may be waived at any time by the Party that is entitled to the benefit thereof, but no such waiver shall be effective unless set forth in a written instrument duly executed by or on behalf of the Party waiving such term or condition. The waiver by either Party hereto of any right hereunder or of the failure to perform or of a breach by the other Party shall not be deemed a waiver of any other right hereunder or of any other breach or failure by such other Party whether of a similar nature or otherwise. The rights and remedies provided herein are cumulative and do not exclude any other right or remedy provided by Applicable Law or otherwise available except as expressly set forth herein.
14.11 No Benefit to Third Parties. Except as provided in ARTICLE 11 (Indemnity), covenants and agreements set forth in this Agreement are for the sole benefit of the Parties hereto and their successors and permitted assigns, and they shall not be construed as conferring any rights on any other Persons.
14.12 Further Assurance. Each Party shall duly execute and deliver, or cause to be duly executed and delivered, such further instruments and do and cause to be done such further acts and things, including the filing of such assignments, agreements, documents and instruments, as may be necessary or as the other Party may reasonably request in connection with this Agreement or to carry out more effectively the provisions and purposes hereof, or to better assure and confirm unto such other Party its rights and remedies under this Agreement.
14.13 Performance by Affiliates. Either Party may exercise its rights and perform its obligations under this Agreement directly or through one or more of its Affiliates and each Party’s Affiliates will have the benefit of all rights (including all options and licenses) of such Party under this Agreement. Accordingly, in this Agreement “Xxxxxxx” will be interpreted to mean “Xxxxxxx or its Affiliates” and “Dyadic” will be interpreted to mean “Dyadic or its Affiliates” where necessary to give each Party’s Affiliates the benefit of the rights provided to the applicable Party in this Agreement. Each Party will remain responsible hereunder for the acts and omissions of its respective Affiliates hereunder, and any breach of the terms or conditions of this Agreement by an Affiliate in the performance of such Party’s obligations hereunder will be deemed a breach of this Agreement by such Party.
14.14 Relationship of the Parties. It is expressly agreed that Dyadic, on the one hand, and Xxxxxxx, on the other hand, shall be independent contractors and that the relationship between the Parties shall not constitute a partnership, joint venture or agency, including for all tax purposes. Neither Dyadic, on the one hand, nor Xxxxxxx, on the other hand, shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other, without the prior written consent of the other Party to do so. All persons employed by a Party shall be employees of such Party and not of the other Party and all costs and obligations incurred by reason of any such employment shall be for the account and expense of such Party.
14.15 Counterparts; Facsimile Execution. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. This Agreement may be executed by facsimile or electronically transmitted signatures and such signatures shall be deemed to bind each Party hereto as if they were original signatures.
14.16 References. Unless otherwise specified, (a) references in this Agreement to any Article, Section or Schedule means references to such Article, Section or Schedule of this Agreement, (b) references in any Section to any clause are references to such clause of such Section, and (c) references to any agreement, instrument or other document in this Agreement refer to such agreement, instrument or other document as originally executed or, if subsequently amended, replaced or supplemented from time to time, as so amended, replaced or supplemented and in effect at the relevant time of reference thereto.
14.17 Schedules. In the event of any inconsistencies between this Agreement and any schedules or other attachments hereto, the terms of this Agreement shall control.
14.18 Construction. Except where the context otherwise requires, wherever used, the singular shall include the plural, the plural the singular, the use of any gender shall be applicable to all genders and the word “or” is used in the inclusive sense (and/or). Whenever this Agreement refers to a number of days, unless otherwise specified, such number refers to calendar days. The captions of this Agreement are for convenience of reference only and in no way define, describe, extend or limit the scope or intent of this Agreement or the intent of any provision contained in this Agreement. The term “including,” “include” or “includes” as used herein means “including, but not limited to,” and shall not limit the generality of any description preceding such term. The word “will” will be construed to have the same meaning and effect as the word “shall.” The word “notice” means notice in writing (whether or not specifically stated) and will include notices, consents, approvals and other written communications contemplated under this Agreement. Any reference herein to any person or entity will be construed to include the person’s or entity’s successors and permitted assigns. The language of this Agreement shall be deemed to be the language mutually chosen by the Parties and no rule of strict construction shall be applied against either Party hereto. Each Party represents that it has been represented by legal counsel in connection with this Agreement and acknowledges that it has participated in the drafting hereof. In interpreting and applying the terms and provisions of this Agreement, the Parties agree that no presumption shall apply against the Party which drafted such terms and provisions.
[SIGNATURE PAGE FOLLOWS.]
THIS AGREEMENT IS EXECUTED by the authorized representatives of the Parties as of the Effective Date.
|
XXXXXXX BIOTECH, INC. |
|
|
By:
Name:
Title: |
By:
Name:
Title: |
[Signature Page to Research, License, and Collaboration Agreement]
Schedule 1.40
Danisco License Agreement
See attached.
Schedule 1.51
Examples of Dyadic Assigned C1 Rights
The foregoing are non-limiting examples of the allocation of ownership and rights relating to the definition of Dyadic Assigned C1 Rights. The term Xxxxxxx shall mean Xxxxxxx, its Designee and any Third Party who accesses Dyadic’s Know-How or Dyadic’s Materials.
Example 1:
[***].
Example 2:
[***].
Example 3:
[***].
Schedule 1.52
Dyadic Background Patent Rights
[***]
Schedule 1.102
Initial Xxxxxxx Proteins
[***]
Schedule 1.103
Initial Reserved Targets
[***]
Schedule 1.119
Xxxxxxx & Xxxxxxx Universal Calendar
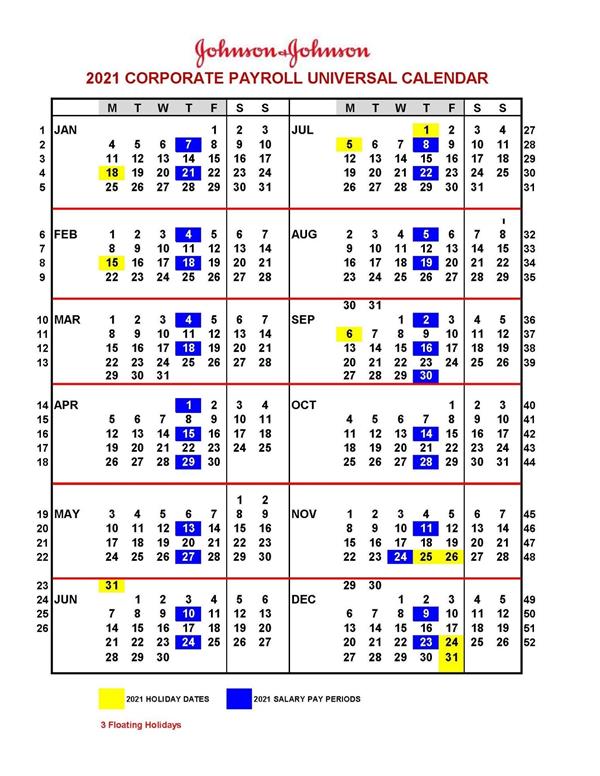
Schedule 2.1.1
Initial Research Plan
See attached.
Schedule 5.7
Approved Subcontractors
Contract research organizations who will conduct the initial research work under the work plan and who will optimize the DNA sequences and who Dyadic will order synthetic fragments for the target proteins:
[***]
Schedule 5.9.1
Initial Technology Transfer: Specification
[***]
Schedule 9.6
Press Release
See attached.
Schedule 10.2
Dyadic Disclosure Schedule
NONE
Schedule 10.2.1
Existing Patents
See Schedule 1.52.
Schedule 10.2.4
Existing In-Licenses
[***]
