LICENSE AGREEMENT by and between MGI PHARMA, INC. and CILAG GmbH INTERNATIONAL
RECITALS |
1 | |||
ARTICLE 1 DEFINITIONS |
1 | |||
ARTICLE 2 LICENSE |
11 | |||
2.1 License |
11 | |||
2.2 Sublicenses and Affiliates |
11 | |||
2.3 No Conflict |
12 | |||
2.4 Right of First Offer to a Related Product |
12 | |||
ARTICLE 3 PAYMENTS AND MILESTONES |
12 | |||
3.1 Up-Front Payment |
12 | |||
3.2 Milestones |
12 | |||
3.3 Royalty Payments |
12 | |||
3.4 Discounting |
13 | |||
3.5 Bundling |
13 | |||
3.6 Other |
13 | |||
ARTICLE 4 JSC, JOCs, COMMERCIALIZATION AND
DEVELOPMENTS PLANS |
13 | |||
4.1 Development and Commercialization Plans |
13 | |||
4.2 Plan Contents |
14 | |||
4.3 Cooperation and Efforts |
15 | |||
4.4 Joint Steering Committee |
15 | |||
4.5 Project Leaders |
16 | |||
4.6 Joint Operating Committees |
16 |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
i
ARTICLE 5 OBLIGATIONS OF THE PARTIES |
17 | |||||
5.1 |
General | 17 | ||||
5.2 |
Development | 17 | ||||
5.3 |
Research and Development Support | 18 | ||||
5.4 |
New Indications | 19 | ||||
5.5 |
Additional Trials | 21 | ||||
5.6 |
Delivery of Information | 21 | ||||
5.7 |
Regulatory Responsibilities | 22 | ||||
5.8 |
Safety Reporting | 23 | ||||
5.9 |
Licensed Products Supplied to Named Patients | 24 | ||||
5.10 |
Commercialization | 24 | ||||
5.11 |
Voluntary and Mandatory Recalls: Potential Hazards | 25 | ||||
5.12 |
Records and Reports | 26 | ||||
5.13 |
Third Parties | 26 | ||||
| 26 | ||||||
6.1 |
Manufacturing and Supply Agreement | 26 | ||||
6.2 |
Pricing | 30 | ||||
6.3 |
Quality Assurance | 30 | ||||
6.4 |
Assignment of Existing Supply Agreements | 31 | ||||
ARTICLE 7 PAYMENTS: BOOKS AND RECORDS |
31 | |||||
7.1 |
Milestone Payments | 31 | ||||
7.2 |
Royalty Reports and Payments | 32 | ||||
7.3 |
Payment Method | 32 | ||||
7.4 |
Currency Conversion | 32 | ||||
7.5 |
Taxes | 32 | ||||
7.6 |
Records and Inspection | 33 | ||||
| 34 | ||||||
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
ii
8.1 Ownership of Inventions |
34 | |||
8.2 Patent Prosecution |
34 | |||
8.3 Cooperation in Prosecution |
35 | |||
8.4 Patent Term Extensions |
35 | |||
8.5 Third Party Infringement Claims |
35 | |||
8.6 Enforcement |
35 | |||
8.7 Patent Marking |
36 | |||
ARTICLE 9 TRADEMARKS |
36 | |||
9.1 Product Trademark |
36 | |||
9.2 License |
37 | |||
9.3 Registration |
37 | |||
9.4 Ownership |
37 | |||
9.5 Recordation |
37 | |||
9.6 Guidelines and Approval |
37 | |||
9.7 Termination |
37 | |||
ARTICLE 10 CONFIDENTIALITY |
38 | |||
10.1 Non-Use and Non Disclosure |
38 | |||
10.2 Exclusions |
38 | |||
10.3 Permitted Use and Disclosure |
38 | |||
10.4 Prior Non-Disclosure Agreement |
39 | |||
10.5 Terms of the Agreement |
39 | |||
10.6 Publication of Licensed Products Information |
40 | |||
ARTICLE 11 REPRESENTATIONS AND WARRANTIES |
40 | |||
11.1 By MGI |
40 | |||
11.2 By Licensee |
42 | |||
11.3 Disclaimer |
43 |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
iii
ARTICLE 12 INDEMNIFICATION |
43 | |||||
12.1 Indemnification of MGI |
43 | |||||
12.2 Indemnification of Licensee |
44 | |||||
12.3 Procedure |
44 | |||||
12.4 Insurance |
44 | |||||
ARTICLE 13 TERM AND TERMINATION |
45 | |||||
13.1 Term |
45 | |||||
13.2 Termination for Non-Payment and Termination Without Cause |
45 | |||||
13.3 Termination for Material Breach, Change of Control or Termination
SuperGen License |
45 | |||||
13.4 Insolvency of a Party |
48 | |||||
13.5 Termination on a Country-by-Country Basis |
49 | |||||
13.6 Tolling of Cure Period |
49 | |||||
ARTICLE 14 EFFECT OF EXPIRATION OR TERMINATION |
49 | |||||
14.1 Accrued Obligations |
49 | |||||
14.2 Effects of any Expiration or Termination |
50 | |||||
14.3 Rights on Expiration or Termination |
50 | |||||
14.4 Rights |
52 | |||||
ARTICLE 15 MISCELLANEOUS |
52 | |||||
15.1 Article and Section Headings, Language and Construction |
53 | |||||
15.2 Governing Law |
53 | |||||
15.3 Submission to Jurisdiction |
53 | |||||
15.4 Waiver of Jury Trial |
53 | |||||
15.5 Other Remedies |
53 | |||||
15.6 Injunctive Relief |
53 | |||||
15.7 Dispute Resolution |
53 | |||||
15.8 Alternative Dispute Resolution |
54 |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
iv
15.9 |
Jurisdiction | 58 | ||||
15.10 |
No Implied Waivers: Rights Cumulative | 58 | ||||
15.11 |
Independent Contractors | 58 | ||||
15.12 |
Notices | 58 | ||||
15.13 |
Assignment | 59 | ||||
15.14 |
Modification | 59 | ||||
15.15 |
Severability | 59 | ||||
15.16 |
Publicity Review | 60 | ||||
15.17 |
Counterparts | 60 | ||||
15.18 |
Export Laws | 60 | ||||
15.19 |
Limitation of Liability | 60 | ||||
15.20 |
Entire Agreement | 60 | ||||
SCHEDULES: |
61 | |||||
Schedule 1.7 — J&J Universal Calendar — 2006 |
||||||
Schedule 1.18 — Decitabine |
||||||
Schedule 1.51 — Licensed Patents |
||||||
Schedule 1.66 — Orphan Product Designation for Decitabine in EC |
||||||
Schedule 1.80 — License Agreement between SuperGen, Inc. and MGI Pharma, Inc.
|
||||||
Schedule 1.75 — Manufacturing and Supply Agreements |
||||||
Schedule 1.90 — R&D/GCP Audit Plan |
||||||
Schedule 2.0 — Press Release |
||||||
Schedule 2.1 — Example of Currency Conversion |
||||||
Schedule 3.0 — Initial Global Development Plan & Listing of On-going Clinical Trials |
||||||
Schedule 6.1(b) — Minimum Product Order Quantities |
||||||
Schedule 6.1(c) — Licensee Initial Product Forecast |
||||||
Schedule 6.1(e) — Form of Purchase Order |
||||||
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
1
| 1.1. | “Additional Trials” shall have the meaning set forth in Section 5.5(a). | ||
| 1.2. | “Affiliate” shall mean, in the case of a subject entity, another entity that controls, is controlled by or is under common control with the subject entity, but only for so long as such control exists. For purposes of this definition only, “control” shall mean beneficial ownership (direct or indirect) of at least fifty percent (50%) of the shares of the subject entity entitled to vote in the election of directors (or, in the case of an entity that is not a corporation, in the election of the corresponding managing authority); provided that where the local law does not permit foreign equity ownership of at least fifty percent (50%), then “control” shall mean the beneficial ownership (direct or indirect) of the maximum percentage of outstanding stock or voting rights permitted by local law. | ||
| 1.3. | “Agreement” shall have the meaning set forth in the Preamble. | ||
| 1.4. | “AML” shall have the meaning set forth in Section 2.1. | ||
| 1.5. | “API” shall have the meaning set forth in Section 1.28. | ||
| 1.6. | “Bright Stock” shall mean unlabeled finished units of the Licensed Products supplied by MGI to Licensee for labeling and distribution in the Territory pursuant to the terms hereof. | ||
| 1.7. | “Calendar Quarter” shall mean a calendar quarter based on the J&J Universal Calendar for that year, a copy of which, for 2006, is attached hereto as Schedule 1.7, and which shall be updated by Licensee for each Calendar Year of the Term consistent with the J&J Universal Calendar used for Licensee’s internal business purposes; provided, however, that the first calendar quarter for the first Calendar Year shall extend from the Effective Date to the end of the then-current Calendar Quarter and the last Calendar Quarter shall extend from the first day of such Calendar Quarter until the effective date of the termination or expiration of the Agreement. | ||
| 1.8. | “Calendar Year” shall mean a calendar year during the Term based on the J&J Universal Calendar for that year, a copy of which, for 2006, is attached hereto as Schedule 1.7, and which shall be updated by Licensee for each Calendar Year of the Term consistent with the J&J Universal Calendar used for Licensee’s internal business purposes. For the first Calendar Year, the Calendar Year shall begin on the Effective Date and the last day shall be December 31, 2006. The last Calendar Year of the Term shall begin on the first day of the J&J Universal Calendar Year for the year during which termination or expiration of the Agreement will occur, and the last day of such Calendar Year shall be the effective date of such termination or expiration. | ||
| 1.9. | “Change of Control” shall mean, with respect to a Party, any transaction or series of related transactions that constitute: (a) the sale or lease of all or substantially all of |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
2
| such Party’s business or assets to an acquiring entity; (b) any merger, consolidation, share exchange, recapitalization, business combination or other transaction to which such Party is subject resulting in the exchange of the outstanding shares of such Party for securities or consideration issued, or caused to be issued, by the acquiring entity; or (c) an acquiring entity having obtained beneficial ownership of fifty percent (50%) or more of the outstanding voting securities of such Party; unless in any of cases (a), (b) or (c) the stockholders of such Party as of the date prior to the closing date of such transaction or series of related transactions hold more than fifty percent (50%) of the voting securities in the surviving entity in such transaction or its parent outstanding immediately after the closing of such transaction or series of transactions. | |||
| 1.10. | “Claim” shall have the meaning set forth in Section 12.1. | ||
| 1.11. | “CMC” shall have the meaning set forth in Section 1.1054. | ||
| 1.12. | “Commercialization”, “Commercialize” and “Commercial” shall mean activities directed to labeling of Bright Stock, launching, marketing, promoting, detailing, educating, distributing, importing, and selling Licensed Products, together with post-marketing studies within the Territory. | ||
| 1.13. | “Commercialization Plan” shall have the meaning set forth in Section 4.1(a). | ||
| 1.14. | “Company Core Data Sheet” or “CCDS” shall mean the complied documentation relating to the medical and scientific data relating to the Licensed Product, including safety and effective use of Licensed Product, without regard to any specific country regulatory labeling requirements. | ||
| 1.15. | “Competing Product” shall mean any prescription pharmaceutical product other than the Licensed Products that |
| (a) | contains Decitabine or a pro-drug of Decitabine as the active ingredient (unless the pro-drug is an active ingredient in a pharmaceutical product that is being marketed as of the Effective Date of this Agreement), or | ||
| (b) | is an ester, phosphate, solvate, hydrate or tautomer of Decitabine (except for pharmaceutical products containing one or more polynucleosides or polynucleotides comprising at least one base of Decitabine), or | ||
| (c) | are Decitabine Metabolites (except for pharmaceutical products that are approved for uses outside of the field of hematological diseases and hematopoietic disorders); |
| in each of (b) and (c) so long as the active ingredient of such pharmaceutical product remains Decitabine or an active Decitabine Metabolite. | |||
| For purposes hereof, “Decitabine Metabolites” shall mean the compounds resulting from the in vivo metabolic breakdown of Decitabine, including the mono-, di- and tri-phosphates of Decitabine. Notwithstanding the preceding sentence, Decitabine |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
3
| Metabolites shall not include (1) analogs of Decitabine metabolites, or (2) polynucleosides or polynucleotides comprising at least one base of Decitabine, wherein the term “analogs” shall be limited to compounds that are not Decitabine Metabolites. | |||
| 1.16. | “Confidential Information” shall mean all information, data, and technology in any form, disclosed by or on behalf of a Party in connection with this Agreement, including but not limited to assays, chemical structures, pre-clinical and clinical trial results, diagnostics, biological markers, protein structures, mechanisms of action, analytical techniques, chemical synthesis, enzymology, computational models, Manufacturing processes, market information, patent applications, inventions, know-how, trade secrets, knowledge, ideas, developments, prototypes, discoveries, invention disclosures, products, procedures, methods, techniques, materials, instructions, specifications, recipes, designs, formulas, compositions, research, modifications, protocols, works of authorship, business plans, financial projections, and any other information, data, and technical subject matter, whether biological, chemical, analytical, clinical, Manufacturing or quality control related, or otherwise, including pharmacological, toxicological and clinical information and test data, and related reports, statistical analyses, Commercialization plans, expert opinions and the like, the secrecy of which confers a competitive advantage upon that Party. As used herein, Confidential Information shall not include rights under Patent Rights (but does include the subject matter disclosed in non-published applications). | ||
| 1.17. | “Control” or “Controlled” shall mean, with respect to any intellectual property right or other intangible property, the possession (whether by license or ownership, or by control over an Affiliate having possession by license or ownership) by a Party of the ability to grant to the other Party access and/or a license or sublicense as provided herein without violating the terms of any agreement with any Third Party. | ||
| 1.18. | “DACOGEN Trademark” shall me mean the DACOGEN xxxx and accompanying logos associated with it. | ||
| 1.19. | “Decitabine” shall mean the compound identified in Schedule 1.18, and all stereoisomers, salts and polymorphs of the compound identified in Schedule 1.18. Esters and acids of the compound identified in Schedule 1.18 shall be included to the extent they constitute the same active ingredient as the compound identified in Schedule 1.18. | ||
| 1.20. | “Decitabine Metabolites” shall have the meaning set forth in Section 1.15. | ||
| 1.21. | “Delivery(ies)” “Deliver(ed)” shall have the meaning set forth in Section 6.1(g). | ||
| 1.22. | “Dispute Resolution” shall have the meaning set forth in Section 15.7. | ||
| 1.23. | “Develop” and “Development” means the procurement of Regulatory Approvals for Licensed Products in the Territory, preclinical testing, toxicology, clinical trials, quality of life assessments, regulatory affairs, and further activities related to development of Licensed Products to a stage ready for Commercialization thereof, |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
4
| including pre-marketing, launch plans and sales forecasting. It is understood that Development includes ongoing clinical trials of Licensed Products conducted after the initial Regulatory Approval of Licensed Products, including, without limitation, to obtain further Regulatory Approval for expanded labeling or approval for new indications. | |||
| 1.24. | “Development Information” shall have the meaning set forth in Section 5.6(a). | ||
| 1.25. | “Diligent Efforts” shall mean, with respect to each Party’s obligations relating to the Licensed Product in their respective territories, the carrying out of such obligations in a diligent and sustained manner using efforts substantially similar to the efforts such Party devotes to a product of similar market potential, profit potential, similar stage in development or commercialization, based on conditions then prevailing. | ||
| 1.26. | “DR” shall have the meaning set forth in Section 15.8. | ||
| 1.27. | “DR Request” shall have the meaning set forth in Section 15.8(a). | ||
| 1.28. | “Drug Substance” shall mean the active pharmaceutical ingredient (“API”) of Product. | ||
| 1.29. | “Drug Substance Secondary Supplier Price” shall have the meaning set forth in Section 6.1(o). | ||
| 1.30. | “Effective Date” shall have the meaning set forth in the preamble. | ||
| 1.31. | “EMEA” shall mean the European Medicines Evaluation Agency, or any successor thereto. | ||
| 1.32. | “Enforcement Action” shall have the meaning set forth in Section 8.6. | ||
| 1.33. | “Equivalent Pharmaceutical Company” shall have the meaning set forth in Section 13.3(b). | ||
| 1.34. | “Estimated Requirements” shall have the meaning set forth in Section 6.1(a). | ||
| 1.35. | “European Union” or “EU”. shall mean the countries of the European Union, as it is constituted as of the Effective Date and as it may be expanded from time to time. | ||
| 1.36. | “Existing Third Party Agreements” shall mean the SuperGen Agreement, the Trademark Coexistence Agreement, the Pharmachemie Manufacturing Agreement, the Ferro Pfanstiehl Agreement, any all the Manufacturing and supply agreements related to Licensed Product, which agreements are listed on Schedule 1.75, and any other agreements which would effect the rights and obligations of the Parties hereunder. | ||
| 1.37. | “FDA” shall mean the United States Food and Drug Administration. | ||
| 1.38. | “Ferro Pfanstiehl Agreement” shall mean all agreements with Ferro Corporation and its affiliates relating to the API including, but not limited to, the Drug Substance and Validation and Supply Agreement between SuperGen, Inc. and Ferro Pfanstiehl Laboratories, Inc. and its affiliates, effective as of July 2, 2003 and Amendments 1, |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
5
| 2, 3, 4, 5, and 6 thereto, and the Quality agreement between MGI Pharma Inc. and Ferro Corporation, effective February 28, 2006. | ||
| 1.39. | “Field” shall have the meaning set forth in Section 2.1. | |
| 1.40. | “Filling Production Lot” shall mean the minimum product quantity of 10,000 vials. | |
| 1.41. | “Firm Orders” shall have the meaning set forth in Section 6.1(c). | |
| 1.42. | “First Commercial Sale” shall mean the first commercial sale of the Licensed Products in the specified country by or under authority of any of Licensee or its Affiliates following receipt of Marketing Authorization and any required pricing or reimbursement approval of such Licensed Products in such country. Sales for clinical study purposes or compassionate, named patient or similar use shall not constitute a First Commercial Sale. |
| 1.43. | “Forecast” shall mean as defined in Section 6.1(a). | ||
| 1.44. | “FTE” shall mean a full time equivalent person year of professional scientific and/or technical work or managerial work to the extent working on or directly involved in the development according to the Global Development Plan. | ||
| 1.45. | “G5 Countries” shall mean Spain, United Kingdom, France, Germany and Italy | ||
| 1.46. | “Good Clinical Practice” or “GCP” shall mean the then current standards for clinical trials for pharmaceuticals, as set forth in the ICH (as defined below) guidelines and applicable regulations promulgated thereunder, as amended from time to time, and such standards of good clinical practice as are required by the European Union and other organizations and governmental agencies in countries in which a Licensed Product is intended to be sold to the extent such standards are not less stringent than United States GCP. | ||
| 1.47. | “Good Laboratory Practice” or “GLP” shall mean the then current standards for laboratory activities for pharmaceuticals, as set forth in the FDA’s GLP regulations and/or the GLP principles of the Organization for Economic Co-Operation and Development (OECD) and/or the GLP regulations adopted by Japan, as amended from time to time, and such standards of good laboratory practice as are required by the European Union and other organizations and governmental agencies in countries in which a Licensed Product is intended to be sold, to the extent such standards are not less stringent than United States GLP. | ||
| 1.48. | “Good Manufacturing Practice(s)” or “GMP” shall mean 1) the regulatory requirements for current good manufacturing practices promulgated by the United States Food and Drug Administration under the U.S. Food, Drug and Cosmetic Act, 21 C.F.R. § 210 et seq. (“FD&C Act”) and under the Public Health Service Act, Biological Products, 21 C.F.R. §§ 600-610 (“PHS Act”), as the same may be amended from time to time; and 2) such standards of good manufacturing practice as are required by the European Union and other organizations and governmental agencies in countries in which a Licensed Product is intended to be Manufactured or |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
6
| sold, to the extent such standards are not less stringent than those in the United States. | |||
| 1.49. | “Governmental Authority” shall mean any court, tribunal, arbitrator, agency, commission, official or other instrumentality of (i) any government of any country, (ii) a federal, state, province, county, city or other political subdivision thereof or (iii) any supranational body. | ||
| 1.50. | “ICH” shall mean “International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.” | ||
| 1.51. | “Indemnitee” and “Indemnitor” shall have the meaning set forth in Section 12.3. | ||
| 1.52. | “Infringement Actions” shall have the meaning set forth in Section 8.5. | ||
| 1.53. | “Global Development Plan” shall have the meaning set forth in Section 4.1(a). | ||
| 1.54. | “Insolvent” shall have the meaning set forth in Section 13.4. | ||
| 1.55. | “Joint Operating Committee(s)” and “JOC(s)” shall have the meaning set forth in Section 4.6. | ||
| 1.56. | “JOC-C” shall have the meaning set forth in Section 4.6. | ||
| 1.57. | “JOC-D” shall have the meaning set forth in Section 4.6 | ||
| 1.58. | “Joint Steering Committee” or “JSC” shall have the meaning set forth in Section 4.4. | ||
| 1.59. | “Liabilities” shall have the meaning set forth in Section 12.1. | ||
| 1.60. | “Licensed Know-How” shall mean all Regulatory Documentation, Supporting Data, Development information and Confidential Information that is material to the Development and Commercialization of the Licensed Products in the possession of MGI in tangible form as of the Effective Date this Agreement, and reasonably necessary for Licensee to perform its obligations under this Agreement. | ||
| 1.61. | “Licensed Patents” shall mean: (i) the patents and patent applications listed in Schedule 1.51 and any patents issuing therefrom, and all reissues, continuations, continuations-in-part, extensions, reexaminations, and foreign counterparts thereof; and (ii) patents and patent applications MGI or its Affiliates may own or control which are infringed by any of the activities of Licensee performing in accord with this Agreement, including without limitation, all patents and patent applications relating to Product Improvements Controlled by MGI. | ||
| 1.62. | “Licensed Product(s)” shall mean the licensed Product(s). | ||
| 1.63. | “Licensed Product Improvements” shall mean the licensed Product Improvements. | ||
| 1.64. | “Licensee” shall have the meaning set forth in the Preamble. | ||
| 1.65. | “Licensee Additional Development Costs” shall have the meaning set forth in Section 13.3(c)(i). |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
7
| 1.66. | “Licensee Indemnitees” shall have the meaning set forth in Section 12.2. | ||
| 1.67. | “Licensee Invention(s)” shall have the meaning set forth in Section 8.1. | ||
| 1.68. | “MAA” shall mean an application requesting Regulatory Approval for the marketing and/or Commercialization of the Licensed Products for a particular indication in a jurisdiction filed with the relevant Regulatory Authorities in jurisdictions in the Territory. It is understood that MAA does not include applications for pricing or reimbursement approval (“Pricing Approval”). | ||
| 1.69. | “Major Country” shall mean the United Kingdom, France, Germany, Italy, Spain and Japan. | ||
| 1.70. | “Manufacturing” or “Manufacture” shall mean activities directed to producing, manufacturing, processing, filling, finishing, packaging, labeling, quality assurance testing and release, shipping and storage of Product. | ||
| 1.71. | “Manufacturing and Supply Agreement” shall have the meaning set forth in Section 6.1. | ||
| 1.72. | “Marketing Authorization” shall mean, with respect to a particular jurisdiction in the Territory, any authorization which is legally required under applicable laws, regulations, administrative decisions, or otherwise to put a pharmaceutical product on the market or for commercial sale in the jurisdiction for use in treatment of any indication. It is understood that, as used herein, Marketing Authorization does not include pricing or reimbursement approval. | ||
| 1.73. | “MDS” shall have the meaning set forth in Section 2.1. | ||
| 1.74. | “MGI” shall have the meaning set forth in the Preamble. | ||
| 1.75. | “MGI Indemnitees” shall have the meaning set forth in Section 12.1. | ||
| 1.76. | “MGI Invention(s)” shall have the meaning set forth in Section 8.1. | ||
| 1.77. | “NDA” shall mean a New Drug Application and amendments and supplements thereto filed with the FDA. | ||
| 1.78. | “Net Sales” shall mean the gross amount invoiced on the sale of the Licensed Products by Licensee and its Affiliates, and any permitted sublicensees, less reasonable and customary deductions for (a) credits for returns, including withdrawals and recalls; (b) sales rebates and chargebacks; (c) sales, value-added and other taxes; (d) customs duties on sales made by such seller to the customer; but in the case of (a) and (b) only to the extent accrued under applicable United States generally-accepted accounting principles as being deductions that have actually been given to and taken by the customer, and in the case of (c) and (d) only to the extent separately itemized in the invoice to and paid by the customer; and (e) government rebates. If the Licensed Products is sold for consideration other than solely cash, the fair market value of such other consideration shall be included in the calculation of Net Sales, provided that transfers of Licensed Product for use in research and/or |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
8
| Development, including Additional Trials, shall not constitute a Net Sale. For purposes of calculating Net Sales, transfers of the Licensed Products to an Affiliate or licensee for end use by such Affiliate or licensee in a country shall be treated as a sale at the greater of the actual Net Sales and the average Net Sales price for fully arms-length sales of such Licensed Products in such country during the applicable calendar quarter, provided that transfers of Licensed Product for use in research and/or Development, including Additional Trials, shall not constitute a Net Sale. Notwithstanding the foregoing, transfers of Licensed Product for use in research and/or Development, including Additional Trials, where revenue is recognized, as defined by generally accepted accounting principles, by Licensee shall constitute a Net Sale in the amount of the revenue received. | |||
| 1.79. | “On-going Trials” shall mean the clinical trials on-going or complete as of the Effective Date of this Agreement, including but not limited to the trials listed in the Global Development Plan attached as Schedule 3.0. | ||
| 1.80. | “Orphan Product Designations” shall mean the designations of Decitabine as an orphan product by the applicable Regulatory Authority in the Territory. Schedule 1.66 sets for the designation for the European Union. | ||
| 1.81. | “Party” and “Parties” shall have the meaning set forth in the Preamble. | ||
| 1.82. | “Patent Rights” shall mean any and all rights under any of the following: (a) a United States, international or foreign patent, utility model, design registration, certificate of invention, patent of addition or substitution, or other governmental grant for the protection of inventions or industrial designs anywhere in the world, including any reissue, renewal, re-examination or extension thereof; and (b) any application for any of the foregoing, including any international, provisional, divisional, continuation, continuation-in-part, or continued prosecution application, supplemental registrations and extensions thereof, including patent term extensions and restorations and supplemental protection certificates. | ||
| 1.83. | “Pharmachemie Manufacturing Agreement” shall mean all agreements with Pharmachemie B.V. and its affiliates relating to the Licensed Product including, but not limited to, the Manufacturing Agreement between MGI Pharma Inc. and Pharmachemie B.V., effective as of July 6, 2005, and the Quality agreement between MGI Pharma Inc. and Pharmachemie B.V., effective September 8, 2005. | ||
| 1.84. | “Policy Relating to the Employment of Child Labor” shall have the meaning set forth in Section 1.92. | ||
| 1.85. | “Pricing Approval” shall have the meaning set forth in Section 1.68. | ||
| 1.86. | “Product(s)” shall mean (i) the product containing Decitabine which is the subject of MGI’s NDA No. 21-790 as of the date of signing of this Agreement; and (ii) any other pharmaceutical product containing as an active ingredient Decitabine. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
9
| 1.87. | “Product Improvement(s)” shall have the meaning set forth in Section 8.1. | ||
| 1.88. | “Product Trademark” shall mean the Dacogen Trademark and all other Licensed Products specific trademark(s), service xxxx(s), accompanying logos, trade dress and/or indicia of origin under which the Licensed Products are marketed and distributed in accordance with this Agreement. For purposes of clarity, the term Product Trademark(s) shall not include, without limitation, the corporate names and logos of either Party. | ||
| 1.89. | “Project Leader” shall have the meaning set forth in Section 4.5. | ||
| 1.90. | “Public Disclosure” shall have the meaning set forth in Section 15.16 | ||
| 1.91. | “Purchase Order” shall have the meaning set forth in Section 6.1(f). | ||
| 1.92. | “Qualified Manufacturer” shall mean a manufacturer that the Parties agree complies with all applicable national, federal, state and local laws and regulatory requirements, including, but not limited to laws regarding environmental, safety and industrial hygiene, complies with GMP, has the requisite continuity and security of supply necessary to meet forecast demands of the Parties, and complies with Licensee’s Policy Relating to the Employment of Child Labor (“Policy Relating to the Employment of Child Labor”) which is: |
| 1.93. | “Quality Agreement” shall have the meaning set forth in Section 6.3(a), as amended from time to time by the Parties. | ||
| 1.94. | “R&D Payments” shall have the meaning set forth in Section 5.3(a). | ||
| 1.95. | “R&D Services” shall have the meaning set forth in Section 5.3(a). | ||
| 1.96. | “Regulatory Approval” shall mean, with respect to the Product in a particular country, the receipt of Marketing Authorization and price and reimbursement approvals, if required, necessary for sale of the Product in that country as granted by the relevant Governmental Authority(ies) including without limitation the approval of an NDA or MAA. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
10
| 1.97. | “Regulatory Authority(ies)” shall mean, in respect of a jurisdiction, any agency, department, bureau or other governmental entity with authority over the development, Manufacture, use or sale (including approval of NDAs and other XXXx) with respect to the Licensed Products in the jurisdiction, including the FDA and EMEA. | ||
| 1.98. | “Regulatory Documentation” means, with respect to the Product, all filings and supporting documents submitted to any and all Regulatory Authorities relating to such Products, and all data and information contained therein, including any INDs, XXXx (including NDAs), Drug Master Files, investigator’s brochures, correspondence to and from Regulatory Authorities, minutes from teleconferences and meetings with Regulatory Authorities, registrations and licenses, regulatory drug lists, advertising and promotion documents shared with Regulatory Authorities, product labeling, adverse event files, complaint files and Manufacturing records and documentation, including but not limited to materials relating to recalls, field alerts and change controls. | ||
| 1.99. | “Related Product” shall have the meaning set forth in Section 2.4 of the SuperGen License Agreement. In addition, amides and metabolites of Decitabine or of a prodrug of Decitabine that are not Competing Products shall be a Related Product. | ||
| 1.100. | “Safety Data Exchange Agreement” shall have the meaning set forth in Section 5.8(d). | ||
| 1.101. | “Sales Materials” shall have the meaning set forth in Section 5.10(e). | ||
| 1.102. | “Sublicense Agreements” shall have the meaning set forth in Section 2.2. | ||
| 1.103. | “SuperGen” shall have the meaning set forth in the third recital. | ||
| 1.104. | “SuperGen License Agreement” shall mean the Amended and Restated License Agreement between SuperGen, Inc. and MGI Pharma, Inc. effective as of September 21, 2004 and amended as of , 2006, and attached hereto as Schedule 1.80 | ||
| 1.105. | “Supporting Data” shall mean all data and information relating to (a) the pharmacological or toxicological properties of the Products, (b) any pre-clinical or clinical testing and experience in relation to the Products and (c) the chemical composition, synthesis, formulation, compounding, and Manufacturing and quality control testing of the Products, to the extent reasonably required for purposes of any application for Marketing Authorization for the Products. Supporting Data shall also include, but is not limited to, copies of annual reports, integrated study reports, protocols for clinical research and pre-clinical studies, protocol changes and amendments, Chemistry, Manufacturing and Control (“CMC”) sections and amendments, safety data, clinical databases, case report forms and access to patient records, toxicity, safety and metabolism reports and data, and pharmacokinetic data and reports and relating to the Products, as well as, in general, data or information |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
11
| which would typically be part of any submission to FDA or other Regulatory Authority for the purpose of obtaining approval of the Products for any indication. | |||
| 1.106. | “Territory” shall mean all countries of the world with the exception of the United States, Canada and Mexico. | ||
| 1.107. | “Third Party” shall mean any party other than Licensee, MGI and their respective Affiliates. | ||
| 1.108. | “Trademark Coexistence Agreement” shall mean the agreement between SuperGen, Inc. and Dakocytomation Denmark A/S, effective as of February 21, 2005. | ||
| 1.109. | “Wind-down Period” shall have the meaning set forth in Section 14.3(a). | ||
| 1.110. | “Vidaza” shall mean azacitidine (4-amino-1-ß-D-ribofuranosyl-s-trazin-2(1H)-one.) |
| 2.1. | License. Subject to the terms and conditions of this Agreement, MGI hereby grants to Licensee and its Affiliates an exclusive, non-transferable (except as expressly authorized in this Agreement), royalty-bearing license, with the right to sublicense as qualified in Section 2.2 below, under the Licensed Patents, Licensed Products Improvements to the extent Controlled by MGI, and Licensed Know-How to use, develop, import, offer for sale, have sold and sell, the Licensed Products in the Territory, and a non-exclusive license thereunder to make and have made the Licensed Products worldwide, for all diagnostic, preventive and therapeutic uses in the human diseases Myelodysplastic Syndromes (“MDS”) Acute Myelogenous Leukemia (“AML”) and any other indications or uses (the “Field”). MGI also hereby grants Licensee a license to use the Dacogen Trademark as provided for in Section 9 of this Agreement. MGI reserves all rights not expressly granted herein, and no other rights shall be considered granted by MGI by implication, estoppel, reliance, or otherwise. Notwithstanding anything to the contrary, no rights or licenses are granted by MGI in this Agreement with respect to any active ingredient other than Decitabine or with respect to any product other than the Licensed Products. | ||
| 2.2. | Sublicenses and Affiliates. Licensee shall not have the right to sublicense its Commercial rights under Section 2.1 above to any Third Party in (a) Major Countries without MGI’s prior written consent, which shall not be unreasonably withheld (such agreements referred to as “Sublicense Agreements”) and (b) in any country in the Territory without MGI”s prior written consent if such transaction is (i) less than an arms-length transaction, (ii) Licensee has an ownership interest in such Third Party, or (ii) if the Third Party has or is also granted the right to Manufacture Product. Each Sublicense Agreement between Licensee and a sublicensee shall be at least as protective of MGI and all intellectual property licensed hereunder, the Licensed Know-How and other Confidential Information as the terms and conditions of this |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
12
| Agreement, and subordinate thereto, and Licensee shall be responsible to MGI for the performance by each sublicensee as necessary for compliance with the financial and other obligations under this Agreement. Within ten (10) calendar days of entering into a Sublicense Agreement under this Section 2.2, Licensee shall provide MGI with a true, complete and correct copy of such Sublicense Agreement and any amendments thereto. Any attempt to engage a sublicensee other than in accordance with the foregoing shall be null and void. In addition, while Licensee may appoint distributors within the normal channel of distribution in non-Major Countries in the European Union, Licensee may not sublicense any of its other rights under Section 2.1 above to such distributors. | |||
| 2.3. | No Conflict. Subject to the rights expressly reserved by MGI under the provisions of Section 2.1, each Party covenants and warrants that commencing on the Effective Date of this Agreement and continuing until ten (10) years after the date on which the Licensed Product receives Regulatory Approval in the EU, none of such Party or its Affiliates will, directly or indirectly, market, distribute, sell, offer to sell, import, or otherwise commercialize any Competing Products; nor shall such Party or its Affiliates assist, fund, or license or authorize, any Third Party to do any of the foregoing. This provision shall not operate to prevent Licensee or its Affiliates from taking reasonable actions to dispose of a Competing Product. | ||
| 2.4. | Right of First Offer to a Related Product. Should SuperGen provide the right of first offer to a Related Product to MGI under Section 2.4 of the SuperGen License Agreement, and should MGI exercise such right to such Related Product, then MGI shall provide a similar right of first offer to Licensee to Commercialize such Related Product in the Territory under the same procedures described in Section 2.4 of the SuperGen License Agreement. |
| 3.1. | Up-Front Payment. In consideration of the licenses granted to Licensee in this Agreement and upon the terms and conditions contained herein, a nonrefundable upfront payment of $ * shall be made by Licensee to MGI within two (2) days following the Effective Date of this Agreement. | ||
| 3.2. | Milestones. In consideration of the Licenses grated by MGI to Licensee and upon the terms and conditions contained herein, the following nonrefundable Clinical Development Milestones will be made thirty (30) days following notice to MGI of the first occurrence of the following events: |
(a) Upon receipt of EU Regulatory Approval |
$ | * | ||
(b) Upon acceptance of an application for
Japan Regulatory Approval |
$ | * | ||
(c) Upon receipt of Japan Regulatory Approval |
$ | * | ||
(d) Upon annual sales in Japan greater than $* |
$ | * | ||
(e) Upon annual sales in G5 Countries greater than $* |
$ | * |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
13
| The milestone payments set forth above in this Section 3.2(a)-(e) shall each be payable only once and on the first occurrence of the event after the Effective Date of this Agreement. | |||
| 3.3. | Royalty Payments. The royalty payments set forth in Sections 3.3.(a)-(d) below shall apply on a country-by-country basis, for * years following the Effective Date of this Agreement, as follows: |
| (a) | Licensee. Commencing with the First Commercial Sale of the Licensed Product, Licensee shall pay, or cause to pay, to MGI, a royalty equal to * of the Net Sales for the Licensed Products sold by Licensee and its Affiliates in the Territory. | ||
| (b) | Distributors of Licensee. In all countries in the Territory outside of the Major Countries, where Licensed Products are commercialized on Licensee’s behalf through distributors, Licensee shall pay, or cause to pay, MGI a royalty equal to * of Net Sales made by Licensee or its Affiliates to such distributors. | ||
| (c) | Compassionate and Named Patient Sales. Licensee shall at the end of each Calendar Year pay, or cause to pay, to MGI a royalty equal to * of Net Sales of Licensed Products sold in the Territory on a named patient, compassionate use or similar basis, provided that no royalty shall be owed on such sales until such Net Sales exceed $ * per year in the aggregate. | ||
| (d) | Product Sales. If Licensee or its Affiliates make a sale of Products that for whatever reason would not be deemed to be Licensed Products under this Agreement, then Licensee shall pay, or cause to pay, to MGI the royalties set forth in Sections 3.3(a)-(c) above as if such sales were sales of Licensed Products. |
| 3.4. | Discounting. Licensee and its Affiliates shall set prices for the Licensed Products in the Territory in the best interest of the commercial success of the Licensed Products in the Territory. Without limiting the foregoing, if Licensee or its Affiliate sells the Licensed Products to a customer who also purchases other products or services from Licensee or its Affiliates, Licensee agrees not to, and to require that its Affiliates not, discount or price the Licensed Products in a manner that would disadvantage the Licensed Products in order to benefit sales or prices of the other products or services offered for sale by Licensee or its Affiliates to such customer. Without limiting the foregoing obligations, it is understood and agreed that nothing in this Agreement is intended to dictate to Licensee and its Affiliates the resale prices for the Licensed |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
14
| Products in the Territory. If the Licensed Products are sold in combination with other products, or as a kit, then each component of the combination or kit will be considered separately for the purposes of this Section 3.4. | |||
| 3.5. | Bundling. Licensed Products shall be sold in compliance with all applicable laws. | ||
| 3.6. | Other. All payments under this Article 3 shall be non-refundable (except solely in the case of overpayment) and non-creditable (unless otherwise specifically set forth in this Agreement) against other amounts due or payable to MGI under this Article 3 or otherwise under this Agreement. Payments under this Article 3 shall be made in accordance with Article 7 below. |
| 4.1. | Development and Commercialization Plans. |
| (a) | Global Development Plans; Amendments. |
| (i.) | Initial Plan. An initial plan (the first “Global Development Plan”) regarding the global development of the Product and containing all of the On-going Clinical Trials shall be attached to this Agreement as Schedule 3.0. | ||
| (ii.) | Commercialization Plan. No later than *, Licensee shall provide to MGI an opportunity to review and comment upon a plan and budget for the Commercialization of the Licensed Products in the Territory for * (such plans and budgets, together with any updates thereto in accordance with this Agreement, hereinafter referred to as the “Commercialization Plan”). To the extent permitted under applicable law, the Commercialization Plan shall include, with respect to the Commercialization of each Licensed Product in each Major Country: *. The Commercialization Plan shall be updated not less than *, as well as more frequently as reasonably requested by MGI to take into account completion, commencement or cessation of Commercialization activities not contemplated by the then current Commercialization Plan; provided that Licensee shall not be required to update in the Commercialization Plan the budget for Commercialization of a Licensed Product in any Major Country after *. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
15
| Licensee agrees to provide to MGI for its review and comment no later than * in advance of each JSC meeting all interim updates to the Commercialization Plan. Licensee shall reasonably consider MGI’s input and comments. | |||
| (iii.) | Updates. After the Effective Date, the Parties through the JOC-D shall update from time to time the Global Development Plan for the further development of the Products and to take into account completion, commencement or cessation of development activities not contemplated by the then current Plan. | ||
| (iv.) | Input and Comment. Each Party shall reasonably consider the other Party’s input and comments to the Global Development Plan. |
| 4.2. | Plan Contents. |
| (a) | The Global Development and Commercialization Plans shall be consistent with the principle of maximizing the value of the Product on a worldwide basis. To achieve this, the Parties shall cooperate in the review and development of the Plans and budgets. | ||
| (b) | The Global Development and Commercialization Plans and budgets therein shall contain sufficient detail as provided in Section 4.1 (a), and as allowed by applicable laws, with respect to the commercialization and development tactics and other matters to enable the JSC to conduct a meaningful review of such plans. | ||
| (c) | Either Party may also develop and submit to the JOCs from time to time proposed amendments to the Plans. The JOCs shall review and comment on such proposed amendments and submit such substantive amendments to the JSC for review. Subject to each Party performing its respective responsibilities substantially in accordance with the Global Development Plan and the Commercialization Plan, (i) Licensee shall be solely responsible for all decisions regarding the Development and Commercialization of Licensed Products in the Territory, including prices charged for the Licensed Product in the Territory, as well as discounts, rebates and all other deductions from Net Sales in the Territory, and (ii) MGI shall be solely responsible for all decisions regarding development and commercialization of the Product outside the Territory, including the prices charged for the Product in the United States and Mexico, as well as discounts, rebates and all other deductions from net sales in the United States and Mexico. *. |
| 4.3. | Cooperation and Efforts. Each of Licensee and MGI shall use Diligent Efforts to execute and to perform, or cause to be performed, the activities assigned to it relating |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
16
| to the development and commercialization of the Product worldwide and to cooperate with the other in carrying out the development and commercialization of the Product in accordance with the Global Development Plan, the Commercialization Plan and this Agreement, in each case in good scientific manner and in compliance with (a) all applicable national, federal, state and local laws and regulatory requirements, including, but not limited to laws regarding environmental, safety and industrial hygiene, (b) GMP, GCP, and GLP, and (c) Licensee’s Policy Relating to the Employment of Child Labor. MGI shall assist Licensee with obtaining Manufacturing information required for Licensee to obtain Regulatory Approvals in the Territory and fulfill Regulatory reporting obligations from the Third-Party manufacturers. |
| 4.4. | Joint Steering Committee. |
| (a) | Formation. Promptly after the Effective Date, the Parties shall establish a committee (the “Joint Steering Committee” or “JSC”), which shall have overall responsibility for the collaboration established by this Agreement for the development and commercialization activities relating to the Product(s) worldwide. The JSC will consist of three (3) representatives from each Party. Subject to the foregoing, each Party shall have the right to change its members of the JSC by providing written notice to the other. | ||
| (b) | Purposes. The purposes of the JSC shall be (i) to review and comment on the overall development, manufacture and commercialization strategy for the Product worldwide and (ii) to oversee the JOCs and resolve matters brought forth by the JOCs and (iii) resolve disputes in accordance with Sections 15.7 and 15.8. The Parties intend that their respective organizations will use Diligent Efforts to assure success of the collaboration. | ||
| (c) | Responsibilities. In addition to its overall responsibility for the collaboration established by this Agreement, the JSC shall in particular: (i) review and monitor the progress of the Parties’ activities under the Global Development Plan; (ii) review and comment on commercialization and development strategies, the trial protocols related to the Products as submitted by the JOCs; and (iii) review and discuss the Regulatory Documentation, product labeling, and filings. | ||
| (d) | Meetings. The JSC shall meet * each year, unless otherwise agreed by the Parties. JSC meetings may be held in person, through telephone or video conference or other mutually agreeable means, provided that at least one meeting each year shall be held in person. With the consent of the JSC members, other representatives of MGI or Licensee may attend JSC meetings as observers. Each Party shall bear its own personnel and travel costs and expenses relating to JSC meetings. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
17
| (e) | Authority. The JSC shall not have the power to amend or modify this Agreement, and its decisions shall not be in contravention of any terms and conditions of this Agreement. |
| 4.5. | Project Leaders. Licensee and MGI each shall appoint a person (a “Project Leader”) to coordinate and facilitate the exchange of information, including Licensed Know-How and Regulatory Documentation, and communications between the Parties regarding the development and commercialization of the Products worldwide. Each Party shall notify the other within * of the Effective Date of this Agreement of the name and contact information for its Project Leader and shall so notify the other Party (in advance of changing its Project Leader). | ||
| 4.6. | Joint Operating Committees. Promptly after its nomination, the JSC shall establish two joint operating committees “(Joint Operating Committee(s)”) or a JOC focusing on Development (“JOC-D”) and a JOC focusing on Commercialization (“JOC-C”) (the JOC-D and the JOC-C collectively referred to as the “JOCs”), led jointly by two persons, one appointed by each Party. |
| (a) | JOC-D Responsibilities. The JOC-D shall be responsible for reviewing, discussing, overseeing and commenting on the development activities of the collaboration, including annual updates of the Global Development Plan. | ||
| (b) | JOC-C Responsibilities. The JOC-C shall be responsible for reviewing, discussing, overseeing and commenting on the commercialization and manufacturing activities of the collaboration, including annual updates of Plans. | ||
| (c) | Data. The JOCs shall have access, and rights, except as provided for in Sections 5.4 and 5.5 herein, to all clinical trial data related to the Product worldwide. The JOCs may agree to share other data with regard to the Parties’ efforts to develop and commercialize the Products in their respective territories. | ||
| (d) | Audit Rights. Each Party will have the right to audit appropriate records of the other Party to verify expenditures related to the other Party’s development activities. The conduct of such audits shall be in accordance with Section 7.6. | ||
| (e) | Review and Comment. The JOCs shall assist the JSC, as directed, in the JSC’s review of the Parties’ activities. For the avoidance of doubt, the JOCs will have no approval rights over the Global Development Plan or the Commercialization Plan. |
| 5.1. | General. Each Party shall use Diligent Efforts to perform the development and commercialization obligations set forth in this Agreement. All such obligations shall |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
18
| be performed in accordance with the applicable laws, rules and regulations of each country in their respective territories. | |||
| 5.2. | Development. |
| (a) | Clinical Development. Licensee will be solely responsible for Development of the MDS indication and the AML indication for the Licensed Products in the Territory *. Subject to payment by Licensee of the R&D Support described below, MGI will bear the costs of the Development of the Licensed Product for the MDS and AML indications * in the Territory, except that (a) the costs of the Development of the Licensed Product for the MDS and AML indications in Japan shall be solely borne by Licensee; (b) MGI’s costs of Development of the Licensed Product for the MDS and AML indications * in the Territory shall not exceed * including On-going Clinical Trials and modifications or additions thereto; and (c) filing fees associated with applications for Regulatory Approval in the Territory shall be the responsibility of Licensee. Following Marketing Authorization in the EU, MGI shall apply Licensee’s R&D Support payments to the further Development of Licensed Product as determined by the JOC-C and JOC-D *. | ||
| (b) | Clinical Program Locations. If possible, and unless otherwise determined by the JOC, the Parties shall endeavor to conduct the clinical program * at an equivalent number of centers with an equivalent number of patients within the EU and the U.S., unless the cost, speed or outcome of the clinical program will be adversely affected. | ||
| (c) | Compliance with GLP/GCP/GMP. All of the activities of both Parties * shall be done in accordance with (i) all applicable national, federal, state and local laws and regulatory requirements, including, but not limited to laws regarding environmental, safety and industrial hygiene, (ii) GMP, GCP, and GLP, and (iii) Licensee’s Policy Relating to the Employment of Child Labor, as applicable. Licensee is prepared, at Licensee’s sole discretion and expense, to assist MGI, upon MGI’s written request, with regard to GLP, GCP and GMP matters. With respect to any facility or site at which a Party conducts development pursuant to this Agreement, including, where commercially reasonable and within the control of the other Party, third party facilities or sites, each Party shall have the right, at its expense, upon reasonable written notice to the other Party and such third party, and during normal business hours, to inspect such site and facility and any records relating thereto once per year, or more often with cause, to verify the other Party’s compliance with the terms of this Agreement relating to (i) all applicable national, federal, state |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
19
| and local laws and regulatory requirements, including, but not limited to laws regarding environmental, safety and industrial hygiene, (ii) GMP, GCP, and GLP, and (iii) Licensee’s Policy Relating to the Employment of Child Labor. Such inspection shall be subject to the confidentiality provisions of this Agreement. Each Party agrees, to the maximum extent possible, to include in any contract or other written arrangement with a third party relating to such facilities and sites, a clause permitting the other Party to exercise its rights under this Section 5.2. With respect to the On-going Trials and future clinical trials, MGI shall make available for Licensee’s review all audit and inspection reports. The current “R&D/GCP Audit Plan” attached hereto as Schedule 1.90 shall continue to be executed by MGI and any amendments or deviations thereto shall be discussed with Licensee prior to implementation. |
| Licensee shall notify MGI in writing of Licensee’s intent to provide certain research and development support to MGI in the form of R&D Services (“R&D Services” shall mean work performed by Licensee’s or its Affiliates’ employees or contractors directed towards implementing the Global Development Plan). |
| (i.) | For 2006, Licensee shall notify MGI in writing of Licensee’s intent to provide R&D Services no later than July 15, 2006; | ||
| (ii.) | For 2007, Licensee shall notify MGI in writing of Licensee’s intent to provide R&D Services no later than December 15th, 2006; and | ||
| (iii.) | For 2008, Licensee shall notify MGI in writing of Licensee’s intent to provide R&D Services no later than December 15th, 2007. |
| If MGI receives such notification from Licensee, then Licensee and MGI will agree to a work plan for the applicable time period, and apply the Licensee FTE rate of * to the activities to determine the value of the R&D Services to be provided by Licensee during such period. In the event that Licensee does not so notify MGI by any date set out in subparagraphs (a)(i), (a)(ii) or (a)(iii) above, then Licensee shall instead pay MGI a research and development payment (“ R&D Payment”) as follows: |
| (iv.) | the sum of * for work to be performed in 2006; | ||
| (v.) | the sum of * for work to be performed in 2007; and | ||
| (vi.) | the sum of * for work to be performed in 2008. |
| If Licensee and MGI agree that Licensee will provide R&D Services during any applicable year, but the value of R&D Services to be performed during such year are less than the corresponding R&D Payments provided for in Sections 5.3 (b)(i), |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
20
| (b) | R&D Payments. Licensee shall in addition make R&D Payments to MGI as follows: |
| (i.) | two (2) days after the Effective Date of this Agreement, Licensee shall pay MGI *; | ||
| (ii.) | upon the successful completion of the DACO 020 study and associated data analysis, and forty-five (45) days following receipt of the final study report for the DACO 020 study, Licensee shall pay MGI * less any R&D Services performed by Licensee during the year in accordance with that year’s approved R&D Services work plan, such R&D Services not to exceed *; | ||
| (iii.) | upon the successful completion of the DACO 017 study and associated data analysis, and forty-five (45) days following receipt of the final study report for the DACO 017 study, Licensee shall pay MGI * less any R&D Services incurred by Licensee during the year in accordance with that years approved R&D Services work plan, such R&D Services not exceed to *. |
| (c) | All R&D Payments by Licensee to MGI shall be non-refundable when made. |
| 5.4. | New Indications. |
| (a) | Either Party may at any time submit to the JSC a proposal to develop new indication(s) (other than a AML or MDS), dosage amount(s) regimens or dosage forms(s), or other trials intended for regulatory purposes, for the Product. Such proposal shall contain, at a minimum, *. The JSC shall approve such proposal if *. In the event the JSC cannot reach agreement to approve a proposal for a new indication, dosage amount or regimen or dosage form, then: |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
21
| (b) | Licensee may pursue development for the Territory of such new indication, dosage amount or regimen or dosage form at its sole expense, provided, however, that (1) MGI does not object to development of such new indication, dosage amount or regimen or dosage form because it has a reasonable belief, and provides Licensee with its reasoning in writing, that such new indication, dosage amount or regimen or dosage form would create toxicity or drug safety concerns, or (2) because MGI has a reasonable belief, and provides Licensee with its reasoning in writing, that such new indication, dosage amount or regimen or dosage form will be likely to have a negative impact on MGI’s business interest under this Agreement. MGI may elect subsequently to participate in the commercialization of such new indication, dosage amount or dosage form outside the Territory pursuant to the terms of this Agreement, provided that MGI first remits to Licensee an amount equal to * of the development costs incurred by Licensee with respect to such new indication, dosage amount or regimen or dosage form, *. If MGI does not elect subsequently to participate in the commercialization of such new indication, dosage amount or regimen or dosage form outside the Territory, Licensee may not commercialize such new indication, dosage amount or regimen or dosage form outside the Territory. | ||
| (c) | MGI may pursue development outside the Territory of such new indication, dosage amount or regimen or dosage form at its sole expense, provided, however, that (1) Licensee does not object to development of such new indication, dosage amount or regimen or dosage form because it has a reasonable belief, and provides Licensee with its reasoning in writing, that such new indication, dosage amount or regimen or dosage form would create toxicity or drug safety concerns, or (2) because Licensee has a reasonable belief, and provides Licensee with its reasoning in writing, that such new indication, dosage amount or regimen or dosage form will be likely to have a negative impact on Licensee’s business interest under this Agreement. Licensee may elect subsequently to participate in the commercialization of such new indication, dosage amount or dosage form in the Territory pursuant to the terms of this Agreement, provided that Licensee first remits to MGI an amount equal to * of the development costs incurred by MGI with respect to such new indication, dosage amount or regimen or dosage form, *. If Licensee does not elect subsequently to participate in the commercialization of such new indication, dosage amount or |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
22
| regimen or dosage form in the Territory, MGI may not commercialize such new indication, dosage amount or regimen or dosage form in the Territory. |
| 5.5. | Additional Trials. |
| (a) | All clinical trials that are not intended to support, directly or indirectly, a regulatory application but which are instead designed to support the marketing of the Product, shall be referred to as “Additional Trials” hereunder and shall not be supported by R&D Support Payments from Licensee. A Party commencing an Additional Trial shall provide the other Party with the opportunity to review and comment on the trial design. | ||
| (b) | Where both Parties wish to use the results of a given Additional Trial to support marketing or a regulatory application of the Product in their respective territories, the costs of such Additional Trial shall be shared by the Parties. | ||
| (c) | Where one Party does not wish to use the results of any Additional Trial in their respective territory, the other Party conducting the Additional Trial will be responsible for and bear the costs of such trial. If a Party does not contribute to the cost of conducting any Additional Trial hereunder, that Party agrees not to use the results of such trial for any purpose, unless the Parties otherwise agree in writing. Nothing in this Section 5.5(c) shall obligate either Party to pay the cost of conducting any Additional Trial if such Party is obligated to use the results of such trial to fulfill its regulatory obligations hereunder. |
| 5.6. | Delivery of Information. |
| (a) | Promptly after the Effective Date, MGI shall provide to Licensee copies of, or access to (if it is not reasonably feasible to reproduce the referenced subject matter), the NDA and MAA, and the source documents referenced therein (the “Development Information”) following MGI’s receipt thereof, and MGI shall otherwise effect a complete transfer of all Licensed Know-How that is necessary or useful for Licensee in the performance of its obligations or exercise of its rights under this Agreement. This transfer shall occur in an orderly fashion and in a manner such that the value of the transferred information is preserved in all material respects. MGI shall not be considered in breach of this obligation as a result of an inadvertent failure to provide any such subject matter, and Licensee shall endeavor to provide MGI with ongoing guidance on the type of information that will be most useful for Licensee in the performance of its obligations or exercise of its rights under this Agreement. | ||
| (b) | All Licensed Know-How and other Confidential Information obtained from MGI will be used and disclosed by and under authority of Licensee only as may be necessary in performing its obligations and exercising its rights under |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
23
| this Agreement and as may otherwise be agreed by MGI and Licensee in writing. | |||
| (c) | All such disclosure of the Licensed Know-How and other Confidential Information obtained from MGI to a non-governmental Third Party, and use thereof, shall be made under reasonable and customary confidentiality restrictions that are as protective of the MGI and such materials as the terms of this Agreement. | ||
| (d) | Within thirty (30) days of the Effective Date, MGI will provide Licensee with the right to cross reference SuperGen’s IND for the Licensed Products for Licensee’s use in the Development and Commercialization of the Licensed Products in the Territory under this Agreement. Neither Party may use any Licensed Know-How or Confidential Information of the other Party for any purpose outside the Territory, nor shall either Party use Confidential Information of the other Party for any products other than the Licensed Products (and shall not permit or authorize any Third Party to do so) without the prior express written approval of the other Party. |
| (a) | Transfer. Promptly after the Effective Date of this Agreement, (1) MGI shall transfer the previously submitted and withdrawn application for Marketing Authorization for the Licensed Products to Licensee, and (2) shall provide copies of or otherwise make available to Licensee all Regulatory Documentation worldwide existing in draft or final form, including all assessment reports and communications with Regulatory Authorities pre-submission, during review process and post withdrawal, all communication and documents relating to scientific advice and communications with the regulatory consultants. | ||
| (b) | Responsibility. Licensee shall be solely responsible for preparing and filing the Marketing Authorization for the Licensed Products in the Territory and for seeking additional Marketing Authorization for the Licensed Products from the appropriate Regulatory Authorities (including all related Regulatory Documentation), and for all filings, meetings and communications with Regulatory Authorities in connection therewith, including labeling discussions and decisions. | ||
| (c) | Meetings with Regulatory Authorities. Licensee shall be responsible for all interactions with Regulatory Authorities in the Territory. The Parties will be reasonably allowed to participate in meetings with Regulatory Authorities in the other Party’s respective territory, as observers, subject to available space and attendance requirements that may be imposed at such meetings, or which may materially interfere with the priorities or objectives of the non-observer Party at a particular meeting. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
24
| (d) | Regulatory Documentation. Prior to filing with a Regulatory Authority, the filing Party shall forward or otherwise make available to the other Party, any Regulatory Documentation to be filed by the Party or its Affiliates and shall afford the reviewing Party an opportunity to comment on such Regulatory Documentation prior to filing, and shall reasonably consider such comments. Licensee shall, and shall cause its Affiliates to maintain the status of such Marketing Authorization(s). | ||
| (e) | Right to Cross-Reference. Each Party shall have the right to cross-reference and make any other use of the other Party’s Regulatory Documentation for the Product that it would have if it were the owner, including without limitation access to all data contained or referenced in such Regulatory Documentation, in each case as may be reasonably necessary to enable such Party to develop, Manufacture or commercialize the Product; except that such right of cross-reference and use shall not apply to trials pursued independently by one Party pursuant to Sections 5.4 or 5.5. | ||
| (f) | Company Core Data Sheet. Licensee shall own the Company Core Data Sheet (CCDS) once the global adverse event database for the Product has been transferred to Licensee pursuant to Section 5.8. | ||
| (g) | Regulatory Inspections. The Parties shall cooperate in good faith with respect to conduct of any inspections by any Regulatory Authority of a Party’s or contractor’s site and facilities. Subject to MGI’s ability to further and accordingly amend the Pharmachemie Agreement and the Ferro Pfanstiehl Agreements, each Party shall be given the opportunity to attend any inspections by any Regulatory Authority of a Party’s or contractor’s site and facilities, and the summary, or wrap up, meeting with a Regulatory Authority at the conclusion of such site inspection. If such attendance would result in the disclosure to the other Party of confidential information or trade secrets unrelated to the subject matter of this Agreement, the Parties shall enter into a confidentiality agreement covering the unrelated subject matter. MGI warrants to Licensee that MGI shall use commercially reasonable efforts to amend the Pharmachemie Agreement and the Ferro Pfanstiehl Agreements in order to provide the rights provided in this Section 5.7(g). | ||
| (h) | Communications from Regulatory Authorities. To the extent either Party receives written or material oral communication from the FDA or any other Regulatory Authority relating to any activities subject to this Agreement, the Party receiving such communication shall notify the other Party and provide a copy of any written communication, or a summary of oral communications, as soon as reasonably practicable and in no case later than three (3) business days thereafter. |
| 5.8. | Safety Reporting. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
25
| (a) | Prior to First Commercial Sale. Prior to the First Commercial Sale of the Licensed Products in the Territory, MGI shall maintain a global adverse event database for the Product worldwide and shall be responsible for compliance with all applicable laws relating to adverse event reporting and the conduct of clinical trials and reporting obligations relating thereto required by the applicable laws worldwide (including annual safety reports, periodic safety update reports and quarterly line listings). | ||
| (b) | Database Transfer. Prior to the First Commercial Sale of the Licensed Product in the Territory, MGI shall complete the transfer to Licensee, and Licensee shall thereafter maintain a global adverse event database for the Licensed Products and shall generate adverse event reports for the Parties use in their respective territories.. | ||
| (c) | Access. Both Parties shall have access to all data in the global adverse event database. Each Party shall be responsible for submitting adverse events reports to the applicable Regulatory Authorities in the Parties respective territories, except that MGI shall be responsible for submitting adverse event reports to the applicable Regulatory Authorities in the EU until the transfer of the regulatory responsibility provided for herein pursuant to Section 5.7(a). | ||
| (d) | Safety Data Exchange Agreement. Within six (6) months of the Effective Date, but in any event prior to commencement or commercialization of the Licensed Products in the Territory, the Parties will in good faith negotiate and finalize a separate Safety Data Exchange Agreement (the “Safety Data Exchange Agreement”), the terms of which shall set forth the obligations, procedures and timelines for exchange of information pertaining to the safety reporting obligations, including adverse and Serious Adverse Events observed in ongoing clinical trials, and across the global marketplace. The Safety Data Exchange Agreement shall address the timing of and communication responsibilities as the between the Parties to the appropriate Regulatory Authorities. | ||
| (e) | Safety Oversight Working Group. Thirty (30) days after the Effective Date of this Agreement, the Parties shall meet to establish a safety oversight working group (the “Safety Oversight Working Group”) composed of members of both Parties which shall discuss and implement processes and procedures for sharing information needed to support each Party’s respective regulatory responsibilities and as may be necessary for the compliance with the applicable regulatory pharmacovigilance requirements. Any such procedures shall not be construed to restrict either Party’s ability to take action that it deems to be appropriate or required of it under the applicable regulatory requirements, but when permitted by applicable laws and regulations, the Parties shall consult with each other before taking such action. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
26
| 5.9. | Licensed Products Supplied to Named Patients. To the extent permitted by local laws and regulations, and to the extent deemed feasible by Licensee, Licensee shall make the Licensed Products available prior to Regulatory Approval pursuant to a Compassionate Use Program and/or on a Named Patient Basis within the Territory following the FDA approval of the Product for the MDS indication in the U.S. Prior to instituting each such program, Licensee shall establish criteria for inclusion of patients in such program(s), and the Parties must have executed the separate Safety Data Exchange Agreement. | ||
| 5.10. | Commercialization. |
| (a) | Responsibilities. Licensee will have sole responsibility, and shall pay for, sales and marketing activities related to the promotion and distribution of the Licensed Products, including all pre-launch market development and market research activities, *. Licensee shall record all sales of Licensed Products, assume all credit and receivable collection risk, and warehouse and distribute Licensed Product in the Territory. In addition, Licensee shall be responsible for handling all aspects of Licensed Product order processing, invoicing and collection, distribution, inventory, product returns, and receivables. Licensee shall assume all general and physical Licensed Product inventory risks in the Territory. MGI will have sole responsibility, and shall pay for, the promotion and distribution of the Product outside of the Territory, record all sales of Products outside of the Territory, and assume the risks and liabilities for all Product transactions outside of the Territory. | ||
| (b) | JSC Meetings. *. | ||
| (c) | Diligent Efforts. Licensee will use Diligent Efforts to obtain reimbursement and pricing approval for the Licensed Products in each country of the Territory following Marketing Authorization in that country of the Licensed Product, *. Unless otherwise agreed, Licensee will launch the Licensed Products in every Major Country in the Territory wherein it has received Marketing Authorization and pricing approval or reimbursement as the case may be. Notwithstanding the foregoing, should Licensee reasonably consider that it cannot profitably sell the Licensed Products in any country in the Territory, Licensee shall provide MGI with written notice to that effect and Licensee’s reasoning for not entering said country, and both Parties will endeavor to fashion a mutually agreeable solution on how to best maximize the commercial potential for the Licensed Products in such country. Licensee shall have the right to appoint distributors in non-Major Countries within the Territory, and shall notify MGI of such appointments. Licensee shall be responsible for the performance of each such appointed distributor. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
27
| (d) | Sales Force. Licensee shall establish and maintain a high quality training program, and each sales representative shall be trained and informed consistent with Licensee’s requirements. The sales representatives shall be compensated so as to provide sales incentives (or other variable pay components) that are consistent with those provided by Licensee in connection with its other portfolio products of similar sales potential. If MGI notifies Licensee that it believes certain of Licensee’s sales representatives may (i) have violated any laws or regulations or (ii) be damaging relationships with health care professionals or damaging the Licensed Products brand, or (iii) failed to comply with this Agreement, Licensee shall promptly use Diligent Efforts to evaluate and resolve such issue. | ||
| (e) | Sales Materials. Any marketing and promotional materials (“Sales Materials”) created or used by Licensee and its Affiliates for the Licensed Products shall be in compliance with the Regulatory Approval for the Products in the respective country. |
| 5.11. | Voluntary and Mandatory Recalls; Potential Hazards. If either Party believes that a voluntary recall or market withdrawal of the Licensed Products may be necessary in their respective territory, such Party shall notify the other Party within twenty-four (24) hours after it forms such belief. Before taking any action with respect to a recall or market withdrawal, to the extent possible, each Party shall provide the other with a reasonable opportunity to review, comment on and consult with respect to such recall or market withdrawal, and shall consider in good faith any comments or suggestions. Notwithstanding the foregoing, depending on the severity of the situation, either Party may use its discretion to initiate a recall at any time after notifying the other Party, if in its opinion, the recall is needed to protect the Party’s consumer’s safety or the Party’s reputation. As between the Parties, Licensee shall be solely responsible for all recalls and market withdrawals in the Territory. As between the Parties, all recalls and market withdrawals in the Territory shall be at the sole cost and expense of Licensee, except that MGI shall be responsible for costs and expenses of recalls and market withdrawals to the extent they are caused by any breach by MGI of its obligations hereunder to supply Licensed Products to Licensee. Each Party shall inform the other Party as soon as possible but no later than within twenty-four (24) hours of its receipt of any information that (i) indicates or suggests a potential material liability for either Party to a Third Party in the Party’s respective Territory arising in connection with the Licensed Products; (ii) concerns suspected or actual tampering or contamination or other material problems in the Party’s respective Territory with respect to the Licensed Products; (iii) is reasonably likely to lead to a recall or market withdrawal in the Party’s respective Territory of the Licensed Products; or (iv) concerns any ongoing or potential Regulatory Authority investigation, inspection, detention, seizure or injunction in the Party’s respective Territory involving the Licensed Products, including the receipt of any warning |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
28
| letter, material untitled letter or any equivalent communication from a Regulatory Authority in the Party’s respective Territory relating to the Licensed Products. | |||
| 5.12. | Records and Reports. |
| (a) | Licensee shall maintain records of the Development and Commercialization of the Licensed Products (or cause such records to be maintained), as applicable, in sufficient detail and in a good scientific manner as will properly reflect all material work done and results achieved in the performance of the Global Development and Commercialization Plans. | ||
| (b) | At each of the JOC meetings, the Parties shall alternate preparing and disseminating meeting minutes to all members of the applicable JOC, said minutes shall include in reasonable detail, for the JOC-D, the progress of Development (including regulatory activities), and for the JOC-C, the Commercialization activities planned and taken, by and under authority of Licensee, and the results thereof. |
| 5.13. | Third Parties. Licensee shall cause its Affiliates and other Third Parties having responsibilities in connection with the Development and/or Commercialization of the Licensed Products to perform their responsibilities in accordance with all applicable terms and conditions of this Agreement. |
| 6.1. | Manufacturing and Supply Agreement. As between MGI and Licensee, MGI shall retain the obligation to manufacture and supply the Licensed Products to Licensee for distribution in the Territory for so long as is required under the SuperGen Agreement, or longer, as requested by Licensee. The Parties intend to negotiate the terms of a separate manufacturing and supply agreement (the “Manufacturing and Supply Agreement”) in good faith, within three (3) months of the Effective Date. The key terms of this agreement will be strongly influenced by the terms of the Pharmachemie Manufacturing Agreement and Ferro Pfanstiehl Agreement, and shall include the following: |
| (a) | Forecasts. |
| (i.) | Initial Forecast. Attached hereto as Schedule 6.1(c) is Licensee’s Initial Forecast for its requirements of Licensed Product and requested Delivery dates for the period beginning on July 1, 2006. | ||
| (ii.) | Quarterly Forecast. Prior to the end of each Calendar Quarter, Licensee shall provide MGI with a statement of its estimated purchase requirements (“Estimated Requirements”) and expected Delivery dates for Licensed Product for the four (4) Calendar Quarters next succeeding such Calendar Quarter (the “Forecast”). The first six (6) months of the Forecast shall constitute commitments to purchase Licensed Product. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
29
| (b) | Purchase Orders. At least ninety (90) calendar days prior to each Delivery, Licensee shall place a Purchase Order for its requirements and requested Delivery dates of Licensed Product for such Calendar Quarter. Purchase Orders shall be placed in amounts equal to the Filling Production Lot sizes, multiplied by whole number increments of the applicable minimum order quantities as set forth on Schedule 6.1(b). Notwithstanding the foregoing, MGI shall use reasonable efforts to comply with any subsequent changes in such Purchase Orders, but shall not be held liable for its inability to do so. | ||
| (c) | Satisfaction of Firm Orders. MGI shall confirm the receipt of each Purchase Order within ten (10) days of its receipt thereof and provide a Delivery date within thirty (30) days of the indicated date within the Purchase Order upon confirmation. When such Purchase Order receipt is confirmed and a Delivery date provided, the Purchase Order shall become a Firm Order (“Firm Order”). Deliveries of Licensed Product shall be made on the date specified in the Firm Order, but in no event more than fifteen (15) business days in advance of the date specified in the Firm Order without Licensee’s prior written approval. MGI shall notify Licensee at least seventy-two (72) hours prior to actual Delivery of Licensed Product if MGI is not Delivering Licensed Product to Licensee on the requested Delivery date. If the actual delivery date for Bright Stock is more than four (4) calendar weeks after the date specified by MGI, the price for all affected Licensed Product shall be reduced by one (1) percent for, and such reduction shall be reflected in the corresponding invoice. | ||
| (d) | Alternative Sources of Supply. In the event that MGI fails to Deliver Licensed Product within sixty (60) days of any Delivery date, including, but not limited to, as a result of a Force Majeure event, MGI and Licensee, will jointly pursue alternative sources of supply. For the avoidance of doubt, MGI shall not terminate the Pharmachemie Agreement if it remains the only source of supply for Licensed Product. | ||
| (e) | Expiration of Ferro Pfanstiehl or Pharmachemie Agreements. In the event that the Ferro Pfanstiehl Agreement or the Pharmachemie Agreement expire or are terminated, then Licensee and MGI shall agree to a mutually beneficial plan to assure the uninterrupted World Wide supply of the Product for use in their respective territories. In such case, Licensee shall have the right, but not the obligation, to manufacture the Product. | ||
| (f) | Terms. Firm Orders will be made on the form of purchase order attached hereto as Schedule 6.1(e), or in such other form as Licensee and MGI shall agree in writing from time to time (a “Purchase Order”), provided that the terms and conditions of this Agreement shall be controlling over any terms and conditions used in any such Purchase Order used in ordering the Licensed Product. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
30
| (g) | Delivery Terms. All orders shall be delivered EXW (Ex-Works Incoterms 2000) to a third party designated by Licensee at the destination and the mode of transportation specified by Licensee in its purchase orders or in any other notice to MGI delivered pursuant to this Agreement (“Delivery”). MGI shall provide to Licensee a xxxx of lading or similar receipt with respect to each Delivery of Licensed Products. MGI shall cause the notification of Licensee in writing upon each shipment of Licensed Products, which shall be Delivered free and clear of any security interests, liens, claims, pledges, or encumbrances of any kind or nature except as such are created by Licensee. | ||
| (h) | Labeling of Licensed Product. Licensee shall order, and MGI shall Deliver, Bright Stock of Licensed Products or labeled Licensed Products that have been secondarily packaged, at Licensee’s discretion hereunder. Licensee shall have the option to label and pack vials. | ||
| (i) | Secondary Source of Finished Product. |
| (i.) | MGI agrees to qualify a secondary source mutually agreed upon by the Parties of supply of finished Products in accord with its obligations under the SuperGen License Agreement, but not to the detriment of Licensee’s efforts to initiate finished product manufacturing from a third party manufacturer of its choosing as of the expiry of the existing Pharmachemie Manufacturing Agreement. In this regard, MGI and Licensee shall collaborate to pursue alternative manufacturing locations for Licensed Product. | ||
| (ii.) | Should Licensee require Drug Substance from Ferro Pfanstiehl for use in the production of Licensed Products at Third Party manufacturers, for which Licensee may have manufacturing responsibility per this Section 6.1(j), MGI is obligated to procure and have tested for release such quantity of Drug Substance. Forecasting for supply of Drug Substance shall be included in the Quarterly Forecast consistent with Section 6.1(a)(ii). Such Drug Substance will delivered EXW (Ex-Works Incoterms 2000). | ||
| (iii.) | In the event that Licensee is the secondary source of Licensed Product, the Parties shall negotiate an appropriate manufacturing and supply agreement and quality agreement. The Parties shall agree upon one set of specifications for the Licensed Products that apply to all territories. |
| (j) | Extensions or Amendments of Ferro Pfanstiehl or Pharmachemie Agreements. MGI agrees not to extend the existing Pharmachemie Manufacturing Agreement or the existing Ferro Pfanstiehl Agreement , and to use commercially reasonable efforts to negotiate, in the best interests of MGI and Licensee, the termination of the Ferro Pfanstiehl Agreement at such time as is reasonably determined by MGI. MGI also agrees not to amend the Pharmachemie Manufacturing Agreement or Ferro Pfanstiehl Agreement |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
31
| without obtaining Licensee’s input on the amendment and obtaining Licensee’s written consent which consent shall not be unreasonably withheld. Notwithstanding the foregoing, neither Party shall be precluded from entering into an agreement with either Ferro Pfanstiehl or Pharmachemie for the manufacture and supply of Product, provided that the Parties shall work together to ensure that each Party has an adequate supply of Product and Drug Substance, following the expiration or termination of the existing Pharmachemie Manufacturing Agreement or Ferro Pfanstiehl Agreements, provided that any such agreement entered into by MGI does not interfere with Licensee’s right to manufacture the Product pursuant to Section 6.1 of this Agreement. To the extent that Licensee has extra Product, Licensee shall not sell such Product to any party other than MGI. |
| (k) | Capacity. MGI shall maintain sources of Licensed Products with an available manufacturing capacity of at least 150% of the aggregate current annual Forecast starting twelve (months) after the initial Regulatory Approval for the Product in Europe. MGI shall issue a letter to Licensee every year on the anniversary of the effective date of the Manufacturing and Supply Agreement certifying such amount of available capacity. MGI and Licensee shall share long-term Forecasts on an annual basis so as to ensure that capacity will remain available for at least three future years. Should a shortage of available Product occur due to MGI’s failure to maintain such available capacity, Licensee’s orders of Licensed Product shall be filled first from the available supply. | ||
| (l) | Force Majeure. In the event of a Force Majeure as defined in the Manufacturing and Supply Agreement between the Parties, Product batches shall be distributed between MGI and Licensee on a pro rata basis based on the previous twelve (12) months of receipt of Product. | ||
| (m) | Effect of Termination of Agreement. In the event of termination of this Agreement and the Manufacturing and Supply Agreement, Licensee shall at its option be permitted to sell Licensed Product then existing in its inventory. | ||
| (n) | Procurement of Drug Substance. MGI will remain responsible to procure all Drug Substance for Licensed Products from Ferro Pfanstiehl in accordance with the terms of the Ferro Pfanstiehl Agreement while in effect. | ||
| (o) | Secondary Source of API. |
| (i.) | No later than six (6) months after the Effective Date of this Agreement, MGI shall name Licensee as the qualified secondary source of Drug Substance under the terms of the Ferro Pfanstiehl Agreement, provided that Licensee is a Qualified Manufacturer. Licensee shall manufacture Drug Substance at terms including transfer, start-up costs through qualification and validation, and price (the “Drug Substance Secondary Supplier Price”), equal to or lower than, and at a capacity equal to or better |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
32
| than the current terms offered by Ferro Pfanstiehl. All transfer and start-up costs incurred by any secondary source of Drug Substance shall remain the obligation of MGI. If Licensee is unable to qualify under this Section 6.1 (o)(i) as the secondary source of Drug Substance, the Parties shall work together to secure a Qualified Manufacturer as the secondary source of Drug Substance. MGI shall allow Licensee or other Qualified Manufacturer of Drug Substance, as the case may be, to produce Drug Substance up to the limit of the allowable percentage under the terms of the Ferro Pfanstiehl Agreement. In the event that Licensee is the secondary source of Drug Substance, Licensee’s production of Drug Substance will be for its own use in the preparation of Licensed Products. Any savings resulting from the lower “Drug Substance Secondary Supplier Price” (excluding cost savings resulting from favorable tax treatment) shall be shared equally by the Parties. |
| (ii.) | In the event that Licensee is the secondary source of Drug Substance, Licensee shall notify MGI of the amount of Drug Substance that it shall produce and use for its own purposes, and MGI shall notify Licensee of the allowable limits of Drug Substance that Licensee can manufacture for use in Licensed Products for sale globally based on the terms of the Ferro Pfanstiehl Agreement. | ||
| (iii.) | Notwithstanding the terms of Section 6.1(o) (i) and (ii), should MGI require Drug Substance from Licensee for its own use or to supplement the amounts required to produce Licensed Products for sale outside the Territory and Licensee is capable of producing such Drug Substance, Licensee shall provide such Drug Substance to MGI. | ||
| (iv.) | In the event that Licensee is the secondary source of Drug Substance, the Parties shall negotiate an appropriate manufacturing and supply agreement and quality agreement. The Parties shall agree upon one set of specifications for the Drug Substance that apply to all territories. | ||
| (v.) | Title. Title to Drug Substance manufactured by Licensee shall remain with Licensee through the manufacture of such Drug Substance into finished Licensed Product. |
| 6.2. | Pricing. Any and all prices paid by Licensee to MGI, or MGI to Licensee, for either Drug Substance or Licensed Products shall be at the Parties’ cost as indicated in invoices generated by either Ferro Pfanstiehl or Pharmachemie or such other applicable third-party source. | ||
| 6.3. | Quality Assurance |
| (a) | Quality Agreement. A quality agreement (the “Quality Agreement”) will be negotiated simultaneously with the Manufacturing and Supply Agreement between the Parties, and be appended thereto. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
33
| (b) | Release of Licensed Products. Any and all shipments of Licensed Products shall be approved and released for Delivery by Licensee’s Quality Assurance personnel under the terms of the Quality Agreement. Such requirements for release may include review of batch records or other pertinent information as requested by Licensee. | ||
| (c) | Compliance with Applicable Laws. MGI shall warrant the compliance with (i) all applicable national, federal, state and local laws and regulatory requirements, including, but not limited to laws regarding environmental, safety and industrial hygiene, (ii) GMP, and (iii) Licensee’s Policy Relating to the Employment of Child Labor of Pharmachemie and Ferro Pfanstiehl and all suppliers and vendors of Pharmachemie and Ferro Pfanstiehl, and shall provide written credentials attesting to such compliance at Licensee’s request. | ||
| (d) | Audits. Subject to MGI’s ability to further and accordingly amend the Pharmachemie Agreement and the Ferro Pfanstiehl Agreements: (i) Licensee shall have the right to audit Pharmachemie and Ferro Pfanstiehl, and any successor suppliers thereto, as well as MGI for the purposes of regulatory compliance, including those of applicable environmental, health, safety, youth employment, quality system and other governmental regulations of compliance; (ii) Licensee may have up to two individuals for a period of up to three days attend such audits one time per year, or more frequently for cause (i.e., “for cause” being defined as any instance or reasonable suspicion of existing or imminent violation of the applicable laws of Section 6.3(c) as demonstrated by reasonably detailed documentation); (iii) Based on such audits, Licensee and MGI shall together issue a list of observations within thirty (30) days that audited site personnel must respond to within thirty (30) days with corrective action plans, and such list shall include all observations provided by Licensee to MGI; (iv) MGI shall require that audited site personnel implement corrective actions within thirty (30) days, or if not possible, a schedule must be agreed to between MGI, the audited site and Licensee for closure of such corrective action(s); and (v) Failure to respond to such observations or close same shall be considered a material breach of the applicable supply agreement(s), allowing Licensee to immediately pursue alternate sources of supply. In the event that MGI and Licensee disagree on the nature or extent of observations or corrective action plans hereunder, they shall be resolved in accordance with Sections 15.7 and 15.8 of this Agreement. MGI warrants to Licensee that MGI shall use commercially reasonable efforts to amend the Pharmachemie Agreement and the Ferro Pfanstiehl Agreements in order to provide the rights provided in this Section 6.3(d). |
| 6.4. | Assignment of Existing Supply Agreements. MGI warrants to Licensee that MGI has, or will uses commercially reasonable efforts to obtain and shall thereafter maintain the right, to assign the Manufacturing and supply agreements related to |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
34
| Product to Licensee pursuant to Sections 13.3(d) and 13.3(g) of this Agreement, or to SuperGen pursuant to Section 15.3.4 of the SuperGen License Agreement. |
| 7.1. | Milestone Payments. Milestone payments payable by Licensee to MGI under Section 3.2 shall be paid in accordance with the terms thereof and this Article 7. | ||
| 7.2. | Royalty Reports and Payments. Commencing with the first sale of the Licensed Products hereunder, Licensee shall provide a written report for each Calendar Quarter to MGI within forty-five (45) days after the end of each Calendar Quarter, stating in each such report, separately for Licensee and its Affiliates, the number, description, and aggregate Net Sales, by country, of the Licensed Products sold during the calendar quarter (or stating that no sales have occurred if such is the case). Concurrently with making each such report, Licensee shall pay to MGI the royalties due in accordance with Section 3.3. | ||
| 7.3. | Payment Method. All payments under this Agreement shall be made by bank wire transfer in immediately available funds in US dollars to an account designated by MGI. Any payments due under this Agreement which are not paid by the date such payments are due under this Agreement shall bear interest to the extent permitted by applicable law at the one-week LIBOR rate as published by British Bankers Association , on the date such payment is due. This Section 7.3 shall in no way limit any other remedies available to MGI. | ||
| 7.4. | Currency Conversion. All amounts set forth in this Agreement, or in any Schedule, are in U.S. Dollars. If any currency conversion shall be required in connection with the calculation of royalties or other payments hereunder, such conversion shall be made using an average of the daily closing mid-rate over the applicable period for foreign currency, in U.S. dollars, as available in Bloomberg screen CURNCY HP (N127) for each relevant Calendar Quarter; Schedule 2.1 provides an example of such currency conversion. | ||
| 7.5. | Taxes. Licensee will make all payments to MGI under this Agreement without deduction or withholding except to the extent that any such deduction or withholding is required to be made on account of Taxes (as that term is defined in Section 7.5(d)). |
| (a) | Any Tax required to be withheld on amounts payable under this Agreement will promptly be paid by Licensee on behalf of MGI to the appropriate Governmental Authority, and Licensee will furnish MGI with proof of payment of such Tax and such other documentation as shall reasonably be requested by MGI to enable MGI to obtain credit for such Tax against its U.S. Tax liability. Any such Tax required to be withheld will be an expense of and borne by MGI. Licensee will give notice of its intention to begin withholding any such Tax in advance and cooperate to use reasonable and legal efforts to reduce such Tax on payments made to MGI hereunder. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
35
| (b) | Licensee and MGI will cooperate with respect to all documentation required by any government taxing authority or reasonably requested by Licensee or MGI to secure a reduction in the rate of applicable withholding Taxes. | ||
| (c) | If Licensee had a duty to withhold Taxes in connection with any payment it made to MGI under this Agreement but Licensee failed to withhold, and such Taxes were assessed against and paid by Licensee, then MGI will indemnify and hold harmless Licensee from and against such Taxes (including interest, but excluding penalties and additions thereto.) If Licensee makes a claim under this Section 7.5(c), it will comply with the obligations imposed by Section 7.5(b) as if Licensee had withheld Taxes from a payment to MGI. | ||
| (d) | Solely for purposes of this Section 7.5, “Tax” or “Taxes” means any present or future taxes, levies, imposts, duties, charges, assessments or fees of any nature (including interest, penalties and additions thereto) imposed by the applicable Governmental Authority.. |
| (a) | Licensee shall keep and maintain true and complete records setting forth the gross sales of the Licensed Product in each country in the Territory, and of all matters relating to the computation of the Net Sales of the Licensed Product in each country in the Territory, including itemization of all deductions permitted pursuant to Section 1.77 and taken in calculating Net Sales, for a period of five (5) years following such sales. | ||
| (b) | Such records shall be open to inspection, at the written request of MGI with (30) thirty days written notice, and during the normal office hours of Licensee(but not more frequently than once per year unless non-compliance is identified) by a nationally recognized independent certified public accountant or equivalent thereof selected by MGI and reasonably acceptable to Licensee, and retained solely for the purpose of auditing the same at MGI expense. | ||
| (c) | Such audit shall be conducted exclusively for the purpose of verifying the accuracy of reports delivered by Licensee to MGI pursuant to this Section and the accuracy of Licensee determination of the amounts payable or paid by Licensee to MGI hereunder. The accountant shall sign a confidentiality agreement prepared by Licensee and shall then have the right to examine the records kept pursuant to this Section and report to MGI the findings (but not the underlying data) of such examination of records. The accountant shall provide a draft copy of the report to MGI and Licensee for review and comment, and each of MGI and Licensee shall have thirty (30) days after receipt of that report to review and comment on the report which comments shall be provided to the accountant and to each other. The final report shall be provided simultaneously to MGI and Licensee by the independent certified public accountant or equivalent thereof within twenty (20) days after the accountant’s receipt and consideration of such comments. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
36
| (d) | If the result of the audit is contested by either party, then they agree to retain a mutually acceptable independent certified public accounting firm or equivalent thereof within 30 (thirty) days to review the relevant books and records and to submit to the parties its written determination as to the amount in dispute and the basis for its determination. The determination by the accounting firm will be binding on the parties absent manifest error. Each party shall pay one-half of the fees and expenses of the accounting firm for such determination. | ||
| (e) | Upon final determination, if the review reveals a deficiency in the calculation of payments resulting from any underpayment by Licensee, Licensee shall promptly pay MGI the amount remaining to be paid (plus interest based on the one week LIBOR rate as published by British Bankers Association on the date such payment is due), and if such underpayment is by five percent (5%) or more for the period examined, Licensee shall also pay all reasonable costs and expenses of the uncontested audit. If the accountant determines that Licensee has overpaid MGI Licensee shall deduct such overpayment on the next payment to MGI. |
| 8.1. | Ownership of Inventions. As between the parties, MGI shall be the sole owner of all right, title, and interest in and to all technology, inventions, trade secrets, Patent Rights, and other intellectual property rights: (a) in new or improved compositions, new or improved formulations, and new or improved methods of manufacture or use of Decitabine (collectively, the “Product Improvements”), regardless of by whom made or conceived; (b) made and conceived solely by MGI personnel in connection with this Agreement; and (c) made and conceived jointly by personnel of MGI and Licensee in connection with this Agreement and relating directly to Product Improvements. The intellectual property described in (a), (b) and (c) shall be collectively referred to as the “MGI Inventions.” All right, title, and interest in and to all technology, inventions, trade secrets, Patent Rights, and other intellectual property rights made and conceived solely by Licensee personnel in connection with this Agreement (“Licensee Inventions”) other than Product Improvements as described above, shall be owned solely by Licensee. Licensee and its Affiliates shall treat all MGI Inventions as the Confidential Information of MGI pursuant to the terms of this Agreement, and shall not make any disclosure or use thereof without MGI’s prior written consent. MGI shall treat all Licensee Inventions as the Confidential Information of Licensee pursuant to the terms of this Agreement, and shall not make any disclosure or use thereof without Licensee’s prior written consent. Licensee and its Affiliates hereby irrevocably assign, and shall assign and cause its Affiliates to assign, to MGI all right, title, and interest Licensee or its Affiliates may have in and to the MGI Inventions worldwide, including, as between the parties, the right to file, prosecute, and maintain Patent Rights with respect |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
37
| thereto in all countries and under all treaties. Licensee shall, and shall cause its Affiliates to, execute such documents, render such assistance, and take such other actions as MGI may reasonably request, at MGI’s expense, as may be reasonably necessary to apply for, register, perfect, confirm, defend, and enforce all rights with respect to the MGI Inventions. |
| 8.2. | Patent Prosecution. As between the parties, MGI (and its designees) shall have the first right to conduct all activities with respect to the preparation, filing, prosecution and maintenance (including interferences, re-examinations, reissues, oppositions and the like) of the Patent Rights in the Licensed Patents, of jointly owned Patent Rights, and of Patent Rights with respect to the MGI Inventions. Licensee shall inform MGI, and require that its Affiliates inform MGI, of any and all MGI Inventions. During the Term, at its own cost and expense and subject to SuperGen’s rights under the SuperGen License Agreement, MGI shall file, prosecute, and maintain its patents and patent applications covering the Licensed Products, including, without limitation, any patents or patent applications covering Product Improvements filed during the Term, worldwide. Absent Licensee’s written consent, MGI shall not elect not to file, prosecute or maintain any patents or patent applications covering the Licensed Products in any country in the Territory. | ||
| 8.3. | Cooperation in Prosecution. Upon request in connection with the preparation, filing, prosecution, or maintenance of any Patent Rights in accordance with the foregoing, each Party will reasonably cooperate with the other in connection therewith, without demanding any further consideration therefore, including, without limitation, executing and filing applications, registrations, powers of attorney, oaths and other appropriate documents, providing appropriate consents and/or authorizations, and joining in any administrative or judicial action relating to the prosecution or maintenance of any Patent Rights. In addition, upon request, MGI will provide Licensee with copies of filings, prosecution and maintenance of any MGI Patent Rights as provided in Schedule 1.51 as well as any copies of filings, prosecution and maintenance of any MGI Patent Rights not listed in Schedule 1.51 but which claim or could include claims relating to Licensed Products originating from SuperGen during the Term of this Agreement. | ||
| 8.4. | Patent Term Extensions. The Parties shall cooperate, if necessary and appropriate, with each other in gaining patent term extension (including those extensions available under the Supplementary Certificate of Protection of Member States of the EU and other similar measures in any other country) wherever applicable to Patent Rights in the Parties respective territories.. The Parties shall, if necessary and appropriate, use reasonable efforts to agree upon a joint strategy relating to patent term extensions. All filings for such extensions shall be made by the Party Controlling such patent or, in the case of joint Patent Rights, by the Party responsible for filing, prosecuting and maintaining such Patent Rights in accordance with Section 8.2. In the event that a Party Controlling a Patent Right elects not to file for |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
38
| any extension, said Party shall notify the other Party and the other Party shall have the right, at its option, to file for such extension. |
| 8.5. | Third Party Infringement Claims. If the making, sale, importation, or use of any Products pursuant to this Agreement results in a claim, suit or proceeding by a Third Party alleging patent infringement against MGI or Licensee (or their respective Affiliates) (collectively, “Infringement Actions”), such Party shall promptly notify the other Party hereto in writing. Subject to Article 12, the Party subject to such Infringement Action shall have the exclusive right to defend and control the defense thereof (including the conclusion of a potential settlement, but limited to the rights granted pursuant to this Agreement) using counsel of its own choice, and, also subject to Article 12, the Infringement Action shall be at such Party’s own expense; provided, however, that the Party not responsible under the foregoing for defending such Infringement Action may participate in the defense and/or settlement thereof at its own expense with counsel of its choice. The Party defending the Infringement Action agrees to keep the other Party hereto reasonably informed of all material developments in connection with any such Infringement Action. Licensee agrees not to make admissions regarding infringement by the Products of any Patent Rights outside of the Licensed Patents, without obtaining the prior written consent of MGI. Nothing in this Section 8.5 shall be construed as limiting the obligations of indemnification under Article 12. | ||
| 8.6. | Enforcement. Subject to the provisions of this Section 8.6, in the event that Licensee reasonably believes that any of the Licensed Patents in the Territory are infringed or misappropriated by a Third Party or is subject to a declaratory judgment action in the Territory arising from such infringement, in each case with respect to the manufacture, sale or use by a Third Party of a Licensed Product within the Territory or of a Competing Product within the Territory, Licensee shall promptly notify MGI. In such event, as between the Parties, MGI shall have the first right (but not the obligation) to enforce the Licensed Patents with respect to such infringement, or defend any declaratory judgment action with respect thereto (for purposes of this Section 8.6, an “Enforcement Action”) in the Territory. In the event that MGI does not elect to enforce Licensed Patents in a country in the Territory within one hundred-twenty (120) days of a request by Licensee to initiate such enforcement action, Licensee, with the previous consent in writing of MGI, may elect to bring an Enforcement Action. MGI shall have the option to contribute to such Enforcement Action by paying to Licensee shall pay one third (1/3) of the costs and expenses (including attorneys’ and professional fees) incurred by Licensee in connection with such Enforcement Action. In the event MGI and Licensee join or are joined as party plaintiffs to an Enforcement Action, each Party shall bear its own costs related to such Action. Any recovery received as a result of any Enforcement Action to enforce Licensed Patents pursuant to this Section 8.6 by either Party shall be used first to reimburse the Parties for the costs and expenses (including attorneys’ and professional fees) incurred by each in connection with such Enforcement Action, and |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
39
| the remainder of the recovery shall be shared (to the extent the same represents damages from sales of the Licensed Products within the Territory) between the Parties; provided, however, that if Licensee initiates the Enforcement Action and MGI does not agree to pay one third (1/3) of the costs and expenses incurred by Licensee therein, or does not join with Licensee as a party plaintiff, MGI shall not be entitled to any amount recovered in such Enforcement Action. Both Parties shall lend reasonable assistance to the other Party in the enforcement of Patent Rights. |
| 8.7. | Patent Marking. Licensee agrees to xxxx and have its Affiliates xxxx all patented Licensed Products they sell or distribute pursuant to this Agreement in accordance with the applicable patent statutes or regulations in the country or countries of manufacture, use, importation and sale thereof sufficient to preserve all rights and remedies under the Licensed Patents. |
| 9.1. | Product Trademark. Licensee shall have the right and license (as recited in Section 9.2) to distribute, promote, market and sell Licensed Product in the Territory under Product Trademark(s). Licensee or its Affiliates shall have the right, subject to MGI’s prior written approval, not to be unreasonably withheld, to select, register, own and use (and bear all costs associated with) any and all lawful trademarks other than the Dacogen Trademark in connection with such distribution, promotion, marketing and sale of the Licensed Products in the Territory. As between the Parties, it is agreed and understood that, if commercially reasonable, that certain Licensed Product which (as of the Effective Date) is the subject of MGI’s NDA No. 21-790 shall be distributed, promoted, marketed and sold in the Territory by Licensee in the Territory exclusively under the Dacogen Trademark; the Parties agree that if the Dacogen Trademark is not legally available in any one or more of the Major Countries then it shall not be commercially reasonable to use the Dacogen Trademark in any country in the Territory. | ||
| 9.2. | License. MGI hereby grants to Licensee a royalty-free license to use the Dacogen Trademark, to the extent owned or licensed by MGI, and any other Product Trademark(s) owned or licensed by MGI, during the term of this Agreement solely in connection with marketing, promoting, distributing and selling the applicable Licensed Products in the Territory in accordance with this Agreement during its Term. | ||
| 9.3. | Registration. Each Party agrees to file, register and maintain registrations for the Product Trademarks to the extent owned by such Party as appropriate in such Party’s discretion, for the term of this Agreement, for use with the Licensed Products. The costs of filing and maintaining such registrations, and for recordations under Section 9.5 below, in the Territory for the Product Trademark shall be borne by the Party that owns such Product Trademark. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
40
| 9.4. | Ownership. Licensee hereby acknowledges SuperGen’s exclusive ownership rights in the Dacogen Trademark, and accordingly agrees that MGI shall have a right to terminate this Agreement under Section 13.3(b) if, during or after the term of this Agreement, Licensee or its Affiliates challenge or assist others to challenge the Dacogen Trademark, or the registration thereof, or attempt to register any trademarks, marks or trade names confusingly similar to the Product Trademark without MGI’s written consent. | ||
| 9.5. | Recordation. In those countries where a trademark license must be or is desired by MGI or SuperGen to be recorded for a Product Trademark owned by MGI or SuperGen, MGI or SuperGen (as the case may be) will provide and record a separate trademark license for such Product Trademark, and the expense thereof shall be shared equally by MGI or SuperGen, as the case may be, and Licensee. Additionally, the Parties shall enter into and record registered user agreements, as requested by MGI or SuperGen, for Product Trademarks owned by MGI or SuperGen. Licensee shall cooperate in the preparation and execution of such documents. | ||
| 9.6. | Guidelines and Approval. To the extent that the Product Trademark used by Licensee is the Dacogen Trademark, all representations thereof that Licensee and its Affiliates intend to use shall first be submitted to MGI for approval of design, color, and other details or shall be exact copies of those used by MGI and shall in any event comply with usage guidelines as communicated by MGI from time to time. Additionally, representative promotional materials, packaging and Licensed Products that use the Dacogen Trademark shall be submitted to MGI by Licensee for MGI’s reasonable approval prior to their first use and prior to any subsequent change or addition to such promotional materials, provided that if MGI has not responded within twenty (20) business days after such submissions, MGI’s approval will be deemed to have been received. MGI shall have the right to monitor the quality of Licensed Products for which the Dacogen Trademark is used, as reasonably requested from time to time. | ||
| 9.7. | Termination. Licensee’s right to use the Dacogen Trademark shall terminate in each country of the Territory in which Licensee’s rights to distribute the Licensed Products expire or are terminated in accordance with this Agreement. Licensee shall cooperate in the cancellation of any trademark licenses, and registered user agreements, recorded or entered into in such countries and relating to the Dacogen Trademark. In the event of termination or expiration of this Agreement, Licensee shall provide for the complete transfer and assignment of all Product Trademarks Controlled by Licensee and used for sale of Licensed Product and any associated goodwill to MGI at Licensee’s cost and expense, unless such termination is as a result of material breach by MGI. |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
41
| 10.1. | Non-Use and Non-Disclosure. Except as expressly provided herein, the Parties agree that the receiving Party shall not publish or otherwise disclose, and shall not use for any purpose, any Confidential Information of the other Party hereto obtained in connection with this Agreement, and any use and disclosure by or under authority of a Party of the other Party’s Confidential Information in accordance with this Agreement shall be subject to a non-use and nondisclosure agreement comparable to the terms hereof. Each Party shall take the same reasonable measures necessary to prevent any disclosure by its employees, agents, contractors, and consultants of the other Party’s Confidential Information as it applies to the protection of its own Confidential Information. | ||
| 10.2. | Exclusions. Information shall not be considered Confidential Information hereunder if it: |
| (a) | was already in the possession of the receiving Party prior to its receipt from the disclosing Party, as shown by the receiving Party’s books and records (except that this exclusion shall not cause Product Improvements to not be the Confidential Information of MGI); | ||
| (b) | is, or becomes, part of the public knowledge or literature through no fault, act or omission of the receiving Party, provided, Confidential Information shall not be deemed to have entered the public domain merely by reason of its having been filed with any Regulatory Authority; | ||
| (c) | is, or becomes, available to the receiving Party from a source other than the disclosing Party, which source has rightfully obtained the same information and has no obligation of confidentiality with respect to it; | ||
| (d) | is made available on an unrestricted basis by the disclosing Party to a Third Party unaffiliated with the disclosing Party; or | ||
| (e) | is independently developed without use of or reference to the other Party’s Confidential Information. |
| (a) | Notwithstanding the provisions of Section 10.1 above, (i) each Party hereto may use and disclose the other Party’s Confidential Information to the extent such disclosure is reasonably necessary to exercise the rights granted to it, or reserved by it, under this Agreement (including the right to grant sublicenses, as applicable), (ii) each Party hereto may disclose the other’s Confidential Information to the extent such disclosure is reasonably necessary in prosecuting or defending litigation or complying with applicable governmental regulations, submitting information to tax or other governmental authorities, (iii) MGI may disclose Licensee’s Confidential Information to SuperGen, and (iv) Licensee shall be permitted to publicly register (within 21 days of initiating enrollment) in a public trials registry (such as xxx.XxxxxxxxXxxxxx.xxx) any clinical trials in which Licensee is a |
42
| participant, to post the results of all such registered clinical trials to a clinical study results web site within 12 months after trial completion, and/or to publish the results of all such registered clinical trials in a peer-reviewed journal and cite such publications on a public clinical study results web site; provided that in (i) and (iii) above, to the extent possible, the recipient is bound by terms and conditions as protective of the other Party’s Confidential Information as the terms and conditions of this Article 10; provided that in (ii) above, if a Party is legally required to make any such disclosure of the other Party’s Confidential Information, to the extent it may legally do so, it will give reasonable advance written notice to the latter Party of such disclosure and will use its reasonable efforts to secure confidential treatment of such Confidential Information prior to its disclosure (whether through protective orders or otherwise); and further provided that in (iv) above, the information to be submitted for such registration and/or postings shall be treated as a publication in accordance with Section 10.6 and accordingly shall be submitted to MGI at least thirty (30) business days prior to the desired date of registration or posting, except that if MGI requests an additional sixty (60) days in order to prepare and file applications on any Patent Rights contained therein, the Parties agree that such clinical trial shall not commence until after the expiration of the sixty (60) day period. | |||
| (b) | Except as provided in Section 10.3(a), Licensee and its Affiliate shall not disclose information about Product Improvements except to the extent licensed to Licensee hereunder and as required in Licensee’s performance of its obligations hereunder, without MGI’s prior written consent. |
| 10.4. | Prior Non-Disclosure Agreements. Upon execution of this Agreement, the terms of this Article 10 shall supersede any prior non-disclosure, secrecy, or confidentiality agreement between the Parties. Any information disclosed under such prior agreements shall be deemed disclosed under this Agreement. | ||
| 10.5. | Terms of the Agreement. Each of the Parties hereto agrees not to disclose the terms of this Agreement to any Third Party without the prior written consent of the other Party hereto, which consent shall not be unreasonably withheld or delayed, except |
| (a) | to advisors, legal counsel, existing and potential investors and others on a need-to-know basis under conditions which reasonably ensure the confidentiality thereof, | ||
| (b) | as required by any court or other governmental body; | ||
| (c) | as otherwise required by law; | ||
| (d) | in confidence, in connection with the enforcement of this Agreement or rights under this Agreement; | ||
| (e) | in confidence, in connection with a merger, acquisition of stock or assets, proposed merger or acquisition, or the like; |
43
| (f) | as reasonably determined by either Party to be required in connection with any government or regulatory filings, including without limitation filings with the Securities and Exchange Commission, provided that the filing Party consults in good faith with the Party whose Confidential Information is to be disclosed with respect to the specific disclosure as to whether to seek confidential treatment to the extent reasonably practical; and | ||
| (g) | as required to be disclosed in such Party’s financial statements as reasonably determined by such Party. |
| 10.6. | Publication of Licensed Products Information. Neither Party shall publish, publicly present and/or submit for written or oral publication a manuscript, abstract or the like which includes data or other information relating to Products without first providing the other Party with opportunity to review the publication for at least thirty (30) business days prior to submission for publication. The non-Publishing Party shall have the right to delay publication for an additional sixty (60) days in order to prepare and file applications on any Patent Rights prior to submission for publication. Neither Party shall publicly present and/or submit for written or oral publication, a manuscript, abstract or the like which includes Confidential Information of the other Party without the express permission of that other Party. The contribution of each Party shall be noted in all publications or presentations by acknowledgment or co-authorship, whichever is appropriate. |
| 11.1. | By MGI. MGI hereby represents and warrants to Licensee as follows: |
| (a) | MGI has been duly organized and is validly existing as a corporation in good standing under the laws of the state of Minnesota, with corporate power to conduct any lawful business activity. MGI has the corporate power and authority to enter into this Agreement and to consummate the transactions contemplated by this Agreement. | ||
| (b) | The execution, delivery and performance of this Agreement, and the consummation of the transactions contemplated by this Agreement, by MGI have been duly and validly authorized by all requisite corporate action of MGI. This Agreement has been duly executed and delivered by MGI and constitutes the legal, valid and binding obligation of MGI, enforceable against MGI in accordance with its terms, except as enforceability thereof may be limited by bankruptcy, insolvency, reorganization or other similar laws relating to or affecting the rights of creditors generally, and by general principles of equity. | ||
| (c) | The execution, delivery and performance of this Agreement by MGI, and the consummation by MGI of the transactions contemplated by this Agreement, do not in any material respect (i) conflict with or result in any breach of any of |
44
| the provisions of, constitute a default under, result in a violation of, give rise to any right of termination under, or require any authorization, consent (except as may have been obtained), approval, exemption or other action by or notice to any court or governmental body, under the provisions of MGI’s Articles of Incorporation or bylaws or any indenture, mortgage, lease, loan agreement, license or other agreement or instrument to which MGI is a party; or (ii) conflict with or result in the violation of any law, statute, rule or regulation or order, judgment or decree to which MGI is subject. | |||
| (d) | To its actual knowledge, it has sufficient rights to the Licensed Patents, Dacogen Trademark, and the trade secrets in the Licensed Know-How, free and clear of any liens or encumbrances, to grant the licenses to Licensee thereunder in this Agreement. MGI has not assigned and/or granted licenses to the Licensed Patents, the Dacogen Trademark, or the Licensed Know-How, or entered into any inconsistent prior obligations, to any other person or entity that conflict with the rights granted hereunder. | ||
| (e) | Except as provided in the Trademark Coexistence Agreement, and to the best of its knowledge, MGI is the sole and exclusive licensee of all right, title and interest in and to the Dacogen Trademark existing as of the Effective Date, and the Dacogen Trademark is free and clear of any liens, charges and encumbrances. | ||
| (f) | As of the date of signing of this Agreement, there are no actions, suits or proceedings pending or threatened in writing against MGI or, to MGI’s best knowledge and belief having made proper enquiry, SuperGen, at law or in equity before or by any federal, provincial, state, municipal or other governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, which may in any way materially adversely affect the performance by MGI of its obligations under this Agreement or Licensee’s enjoyment of the rights granted to Licensee hereunder and MGI is not aware of any circumstances which might give rise to such actions, suits or proceedings. | ||
| (g) | Prior to the Effective Date MGI has provided Licensee with true, accurate and up to date copies, including all that amend or add to the terms thereof, of the Existing Third Party Agreements existing as of the Effective Date of this Agreement. | ||
| (h) | MGI and SuperGen have amended the SuperGen License Agreement to provide that: (i) SuperGen will consent to the continuation of Licensee’s rights and license granted by MGI under this Agreement under the conditions described in Section 13.3(c) herein, should the SuperGen License Agreement be terminated either by SuperGen for MGI’s breach, provided that such breach was not caused in whole or in part by the Licensee’s breach of this Agreement, or otherwise terminated by MGI; (ii) MGI can assign regulatory |
45
| rights in the Territory, as that term is defined herein, to Licensee and a true and accurate copy of the SuperGen License Agreement as amended is attached hereto as Schedule 1.80. | |||
| (i) | MGI shall not amend the SuperGen License Agreement in any manner that materially and adversely effects Licensee’s rights under this Agreement. | ||
| (j) | To the best of MGI’s knowledge, the Licensed Patents are valid. The Licensed Patents are subsisting and MGI is not aware of any information that might render the Licensed Patents void, invalid, or unenforceable. | ||
| (k) | To the best of MGI’s knowledge, MGI has disclosed to Licensee any data and other information that could reasonably be considered material to the safety or efficacy of the Licensed Products. | ||
| (l) | MGI has disclosed to Licensee in writing prior to the date of signing of this Agreement all of its rights and interests with respect to Competing Products, including all of its plans with respect to Competing Products. It has no rights to Commercialize, and it is not (and does not plan to) Commercialize any Competing Products. |
| 11.2. | By Licensee. Licensee hereby represents and warrants to MGI as follows: |
| (a) | Licensee has been duly organized and is validly existing as a corporation in good standing under the laws of Switzerland, with corporate power to conduct any lawful business activity. Licensee has the corporate power and authority to enter into this Agreement and to consummate the transactions contemplated by this Agreement. No consent, approval or agreement of any person, party, court, government or entity is required to be obtained by Licensee and/or any of its Affiliates in connection with the execution and delivery of this Agreement or the consummation of the transactions contemplated hereby, which has not been obtained. There is nothing in any agreement between Licensee or its Affiliates and any Third Party agreement which, in any way, will limit Licensee’s ability to perform all of the obligations undertaken by Licensee hereunder, such as without limitation its diligence with respect to the Development and Commercialization of the Licensed Products. | ||
| (b) | The execution, delivery and performance of this Agreement, and the consummation of the transactions contemplated by this Agreement, by Licensee have been duly and validly authorized by all requisite corporate action of Licensee. This Agreement has been duly executed and delivered by Licensee and constitutes the legal, valid and binding obligation of Licensee, enforceable against Licensee in accordance with its terms, except as enforceability thereof may be limited by bankruptcy, insolvency, reorganization or other similar laws relating to or affecting the rights of creditors generally, and by general principles of equity. |
46
| (c) | The execution, delivery and performance of this Agreement by Licensee and the consummation by Licensee of the transactions contemplated by this Agreement do not in any material respect (i) conflict with or result in any breach of any of the provisions of, constitute a default under, result in a violation of, give rise to any right of termination under, or require any authorization, consent (except as may have been obtained), approval, exemption or other action by or notice to any court or governmental body, under the provisions of Licensee’s Articles of Incorporation or bylaws or any indenture, mortgage, lease, loan agreement, license or other agreement or instrument to which Licensee is a party, or (ii) conflict with or result in the violation of any law, statute, rule or regulation or order, judgment or decree to which Licensee is subject. | ||
| (d) | During the term of this Agreement, Licensee shall comply, in all material respects with all applicable statutes, rules and regulations of the applicable Regulatory Authorities, with respect to the clinical testing, collection, labeling, storing, testing, or distribution of the Licensed Products, including the applicable corresponding requirement of Regulatory Authorities to the then current “Good Clinical Practice” or GCP regulations, “Good Laboratory Practice” or GLP regulations, “Informed Consent” and “Institutional Review Board” regulations, and all applicable requirements relating to the protection of human subjects for its clinical trials. Licensee shall obtain, or assist MGI in obtaining (as set forth in this Agreement), from the applicable Regulatory Authorities, all requisite permits, approvals, registration and licenses required to Commercialize the Licensed Products prior to marketing or selling the Licensed Products in such country or region. | ||
| (e) | It has disclosed to MGI in writing prior to the date of signing of this Agreement all of its rights and interests with respect to Competing Products, including all of its plans with respect to Competing Products. It has no rights to Commercialize, and it is not planning to Commercialize any Competing Products. |
| 11.3. | DISCLAIMER. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTIES OF ANY KIND EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NONINFRINGEMENT, OR VALIDITY OF ANY PATENTS ISSUED OR PENDING, AND EACH PARTY HEREBY DISCLAIMS SUCH OTHER REPRESENTATIONS AND WARRANTIES. |
47
| 12.1. | Indemnification of MGI. Licensee shall indemnify, defend and hold harmless MGI, its Affiliates, and their respective directors, officers, employees, agents and their respective successors, heirs and assigns (the “MGI Indemnitees”) from and against any and all losses, costs, claims, damages, liabilities and expenses (including reasonable attorneys’ and professional fees and other expenses of litigation) (collectively, “Liabilities”), including Liabilities resulting from a claim, suit, or proceeding (“Claim”) made or brought by a Third Party against a MGI Indemnitee (and further including those arising out of personal injury claims) arising from or occurring as a result of (a) any breach of the representations and warranties made by Licensee in this Agreement; (b) any research, Development, testing, importation, use, offer for sale, sale, marketing, distribution, or other Commercialization of the Licensed Products by or under authority of Licensee or its Affiliates, (c) any act, omission, or failure of Licensee or any of its Affiliates to comply in any material respect, with any applicable laws, regulations and/or administrative decisions relating to the Licensed Products or (d) a Claim by a Third Party as a result of Licensee exercising rights exceeding the scope of the license grant from MGI pursuant to the provisions of Article 2 or arising out of or relating to breach of Articles 8 or 10; except in each case to the extent caused by the breach of contract, gross negligence or willful misconduct of MGI. | ||
| 12.2. | Indemnification of Licensee. MGI shall indemnify, defend and hold harmless Licensee, its Affiliates, and their respective directors, officers, employees, agents and their respective successors, heirs and assigns (the “Licensee Indemnitees”) from and against any and all Liabilities, including Liabilities resulting from a Claim made or brought by a Third Party against an Licensee Indemnitee arising from or occurring as a result of (a) any breach of the representations and warranties made by MGI in this Agreement, (b) any research, Development, testing, importation, use, offer for sale, sale, marketing, distribution, or other Commercialization of the Products by or under authority of MGI or its Affiliates outside of the Territory, (c) any manufacture of the Licensed Products by or on behalf of MGI up to the point that such Licensed Products leaves MGI’s actual or constructive possession and control, (d) any failure of MGI or any of its Affiliates to comply in any material respect with any applicable laws, regulations and/or administrative decisions relating to the Licensed Products, or (e) a Claim by a Third Party as a result of MGI or an Affiliate of MGI exercising rights exceeding the scope of the license grant from SuperGen; except in each case to the extent caused by the breach of contract, gross negligence or willful misconduct of Licensee. | ||
| 12.3. | Procedure. A MGI Indemnitee or Licensee Indemnitee, as the case may be, that intends to claim indemnification (the “Indemnitee”) shall promptly notify the indemnifying Party (the “Indemnitor”) in writing of any Claim in respect of which the Indemnitee intends to claim indemnification under this Article 12. The Indemnitor shall have the sole right to control the defense and settlement of such Claim; provided, that the Indemnitee shall have the right to participate in the defense |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
48
| or settlement of such Claim with counsel of its own choosing at its expense. The Indemnitor shall keep the Indemnitee fully informed of the progress of any such Claim. The indemnity arrangement in this Article 12 shall not apply to amounts paid in settlement of any action with respect to a Claim, if such settlement is effected without the consent of the Indemnitor, which consent shall not be withheld or delayed unreasonably. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Claim, if prejudicial to its ability to defend such action, shall relieve such Indemnitor of any liability to the Indemnitee under this Article 12 but the omission so to deliver written notice to the Indemnitor shall not relieve the Indemnitor of any liability that it may have to any Indemnitee otherwise than under this Article 12. The Indemnitee shall cooperate fully with the Indemnitor and its legal representatives in the investigation of any action with respect to a Claim covered by this indemnification at the expense of the Indemnitor. | |||
| 12.4. | Insurance. Each Party shall procure and maintain at its own cost comprehensive general liability (including coverage for products and maintained throughout the term of this Agreement and for a period of at least five (5) years after the expiration or termination of this Agreement), commercial general liability, product liability, clinical trial liability, and property and casualty insurance providing commercially reasonable coverage (at least * coverage) for its activities under this Agreement and its equipment, premises and businesses. Licensee will maintain foreign local coverage in the Territory where required by foreign law in an amount that, at a minimum, satisfies the legal requirements of that jurisdiction. The insurance policies of the Parties in the United States (as applicable) shall be with financially strong insurance carriers (AM Best Rating of “A: VIII” or higher) and will be primary to any other insurance owned, secured or in place. Licensee shall name MGI as an additional insured on such policies. MGI shall name Licensee as additional insured on such policies. Each Party will furnish to the other Party the corresponding certificates of insurance that evidence the foregoing within fifteen (15) days of the Effective Date. |
| 13.1. | Term. This Agreement shall be effective as of the Effective Date, and unless earlier terminated pursuant to the other provisions of this Agreement, this Agreement shall expire, on a country-by-country basis, upon the later of (a) twenty (20) years after First Commercial Sale of the applicable Licensed Products in the respective country, and (b) the expiration, termination, invalidation, or abandonment of all Patent Rights within the Licensed Patents covering the respective Licensed Products, or the manufacture or use thereof, in the respective country (the “Term”). | ||
| 13.2. | Termination for Non-Payment and Termination Without Cause. |
49
| (a) | In the event a Party fails to pay any undisputed amount due under this Agreement within sixty (60) calendar days following receipt of written notice of such non-payment by the other Party, the other Party may, by written notice to such Party, terminate this Agreement in whole. An amount shall be deemed to be undisputed except to the extent that the Party that is required to make the payment provides the other Party with written notice reasonably describing the amounts disputed, and the reason for the dispute, promptly after receipt of notice of non-payment. | ||
| (b) | Licensee may terminate this Agreement, without cause, in total or on a country-by-country basis after one (1) year following the Effective Date, by providing MGI written notice of such termination ninety (90) days prior to the effective date thereof, provided that in the case of the EU, the Major Countries therein, must be terminated all together. In the event that Licensee exercises its rights under this Section 13.2(b), and Drug Product is being manufactured at the same time for the country or countries being terminated, Licensee shall also be obligated to pay for the costs of such manufacture. | ||
| (c) | Notwithstanding the foregoing, Licensee may terminate this Agreement at any time if the EMEA indicates that an additional clinical trial is required to obtain Marketing Authorization of the Licensed Product for the MDS indication, provided that Licensee provides MGI written notice of its intent to terminate this Agreement under this Section 13.2(c) within ninety (90) days of receipt of the aforementioned indication from the EMEA. Failure of Licensee to notify MGI of its intent to terminate this Agreement under this Section 13.2(c) within ninety (90) days shall constitute a waiver of Licensee’s rights to terminate under this Section 13.2(c). |
| (a) | Without limiting either Party’s ability to terminate in accordance with the other provisions of this Article 13, in the event of a Party’s material breach of this Agreement, the non-breaching Party shall have the right to provide notice of its intention to terminate this Agreement (in whole or in part as set forth in Section 13.5). Such notice shall specify in reasonable detail the facts and circumstances constituting the material breach of this Agreement. Upon the expiration of sixty (60) calendar days after receipt by the breaching Party of such notice, if the breaching Party: (i) has not cured such material breach when it is possible to cure such breach during such 60-day period or (ii) is not diligently pursuing a cure of such breach throughout such 60-day period where it is not possible to cure such breach within such 60-day period, then the non-breaching Party shall be entitled to exercise the rights set forth in Section 13.5. In the event under (ii) above that despite the breaching Party’s efforts to cure such material breach, if such material breach has not been cured within six (6) months following receipt of notice of material breach as |
50
| set forth above by the breaching Party, then the non-breaching Party shall be entitled to exercise the rights set forth in Section 13.5. | |||
| (b) | MGI shall have the right to terminate this Agreement effective upon written notice if: |
| (i.) | Licensee undergoes a Change of Control where the Change of Control results in (1) Licensee or its Affiliates developing and/or commercializing a Competing Product or that product competitive to the Licensed Products with the trade name Vidaza, and (2) Licensee does not agree to divest the same following request from MGI; | ||
| (ii.) | Licensee or its Affiliates acquire or in-licenses a Competing Product or that product competitive to the Licensed Products with the trade name Vidaza, and does not inform MGI in writing within thirty (30) days thereafter of its plans to divest the same, or begins to commercialize the same prior to actually divesting it; | ||
| (iii.) | MGI is provided notice by SuperGen that the SuperGen License Agreement may be terminated as a result of a material breach of this Agreement by Licensee, but only if Licensee does not cure the breach within any applicable cure period under the terms of this Agreement; or | ||
| (iv.) | in accordance with Section 9.4 herein. |
| (c) | If the SuperGen License Agreement is terminated (in whole or in part), either by SuperGen due to the breach of such Agreement by MGI (and such termination is not related to any breach by Licensee of the terms of this Agreement), or otherwise terminated by MGI, then, effective upon the effective date of termination of the SuperGen License Agreement, SuperGen shall promptly enter into an agreement with Licensee on the same terms and conditions of this Agreement,, except as provided in subsections (i), (ii) and (iii) below: |
| (i.) | In no event shall SuperGen be obligated to undertake any development obligation of MGI under this Agreement, provided that Licensee shall have the right to conduct any development that was a pre-existing MGI obligation at its sole cost and expense should SuperGen elect not to perform such activities; however, if following such continuation of Licensee’s rights and license hereunder, SuperGen does not undertake to perform any development of obligation of MGI for which twenty-five percent (25%) of the patients have been enrolled prior to the effective date of termination, then the royalty otherwise payable by Licensee to SuperGen under this Agreement shall be reduced by the actual amount incurred by Licensee or by a third party on Licensee’s behalf (the “Licensee Additional Development Costs”), to perform such development obligations subject to Section 13.3(e); |
51
| (ii.) | following such continuation of Licensee’s rights and license hereunder, SuperGen shall, subject to Section 13.3(f), either (1) supply Licensed Products to Licensee under the terms of this Agreement and the additional Agreements referenced herein (such as the Manufacturing and Supply Agreement and the Quality Agreement) or entered into between MGI and Licensee related to the Product, or (2) shall assign to Licensee all Manufacturing agreements related to Licensee’s receipt of Licensed Products, including the Pharmachemie Agreement and the Ferro Pfanstiehl Agreement and the other agreements listed on Schedule 1.75, in which case Licensee shall supply Licensed Product to SuperGen under terms and conditions at least as beneficial as those under which Licensee was purchasing its supply of Licensed Product under this Agreement, the additional Agreements referenced herein (such as the Manufacturing and Supply Agreement and the Quality Agreement) and any subsequent agreements or entered into between MGI and Licensee related to the supply of Product, such terms and conditions to be embodied in a separate supply agreement which shall be negotiated in good faith by Licensee and SuperGen; and | ||
| (iii.) | Licensee expressly agrees that SuperGen shall assume no losses, costs, liabilities or damages that arise out of or relate to MGI’s performance, non-performance or breach of this Agreement; and | ||
| (iv.) | as between Licensee and SuperGen, the provisions of Section 15.2, 15.3, 15.4 and the first sentence of Section 15.19 hereof shall apply to SuperGen in the same manner such provisions they apply to MGI. |
| (d) | Prior to the assignment of the SuperGen License Agreement by MGI to a successor-in-interest to all or substantially all of the business or assets of MGI pursuant to Section 16.6 of SuperGen License Agreement, MGI shall either (i) obtain the written confirmation of such successor-in-interest that it shall provide Licensee with supply of Licensed Product under the same terms and conditions as under this Agreement, the additional Agreements referenced herein (such as the Manufacturing and Supply Agreement and the Quality Agreement) and any subsequent agreements entered into between MGI and Licensee related to the supply of Product, or (2) assign to Licensee all Manufacturing agreements related to the Licensed Products, including the Pharmachemie Agreement and the Ferro Pfanstiehl Agreement and the other agreements listed on Schedule 1.75, in which case Licensee shall supply Licensed Product to MGI under the terms and conditions of this Agreement and pursuant to a separate supply agreement which shall be negotiated in good faith by Licensee and MGI. | ||
| (e) | If the royalty paid by Licensee is reduced pursuant to Section 13.3(c)(i), then the reduction shall only be in effect until Licensee Additional Development |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
52
| Costs up to * dollars have been recouped through the royalty reduction. | |||
| (f) | In the event that the SuperGen License Agreement is terminated either by SuperGen due to the breach of such Agreement by MGI, and such termination is not related to any breach by licensee of the terms of this Agreement, or otherwise terminated by MGI, then MGI shall assign all Manufacturing agreements related to Licensee’s receipt of Licensed Products to SuperGen, subject to SuperGen’s consent, not to be unreasonably withheld. | ||
| (g) | Change of Control of MGI. In the event of a Change of Control of MGI or the assignment by MGI of this Agreement pursuant to Section 15.13 of this Agreement, MGI shall either (i) obtain the written confirmation of such Change in Control party that it shall provide Licensee with supply of Licensed Product under the same terms and conditions as under this Agreement, the additional Agreements referenced herein (such as the Manufacturing and Supply Agreement and the Quality Agreement) and any subsequent agreements entered into between MGI and Licensee related to the supply of Product, or (2) assign to Licensee all Manufacturing agreements related to Licensee’s receipt of Licensed Products, including the Pharmachemie Agreement and the Ferro Pfanstiehl Agreement and the other agreements listed on Schedule 1.75, in which case Licensee shall supply Licensed Product to MGI, or the successor-in-interest of MGI’s interest in this Agreement, as the case may be, under the terms and conditions of this Agreement and pursuant to a separate supply agreement which shall be negotiated in good faith by Licensee and MGI or Licensee and the successor-in-interest of MGI’s interest in this Agreement, as the case may be. |
| 13.4. | Insolvency of a Party. The Parties shall have the respective rights set forth in this Section 13.4 in the event of the Insolvency of the other Party. |
| (a) | For purposes of this Agreement and this Section 13.4, “Insolvent” shall mean: |
| (i.) | the commencement of a voluntary case or other voluntary proceeding seeking liquidation, reorganization or other relief by a Party with respect to itself or its debts under any bankruptcy, insolvency or other similar law or seeking the appointment of a trustee, receiver, liquidator, custodian or similar official of it or of any substantial part of its property to which this Agreement pertains, or the consent by a Party to any such relief or to the appointment of or taking possession by any such official in an involuntary case or other proceeding commenced against it, or making a general assignment for the benefit of creditors, or, or taking any corporate action to authorize any of the foregoing; has an involuntary case or other involuntary proceeding commenced against it seeking liquidation, |
53
| reorganization or other relief with respect to it or its debts under any bankruptcy, insolvency or other similar law now or hereafter in effect or seeking the appointment of a trustee, receiver, liquidator, custodian or other similar official of it or any substantial part of its property, and such involuntary case or other proceeding remains undismissed and unstayed for a period of ninety (90) days; or an order for relief is entered against such Party under applicable bankruptcy laws; or | |||
| (ii.) | the inability of a Party to pay its debts as they become due and such Party has explicitly or implicitly suspended payment of all or substantially all of its debts as they become due, or in the event that the creditors of such Party take over its management. |
| (b) | Termination in the Event of Licensee’s Insolvency. MGI may terminate this Agreement upon written notice to Licensee in accordance with the provisions of Section 15.12, if, at any time, Licensee is Insolvent as defined in Section 13.4(a). | ||
| (c) | Rights in the Event of Insolvency. The Parties acknowledge and agree that the rights and licenses granted pursuant to this Agreement are, and shall otherwise be deemed to be, for the purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of rights to “intellectual property” as defined under Section 101(35A) of the Bankruptcy Code, and that the Parties, each as a licensee hereunder, shall retain and may fully exercise all of its rights and elections under the Bankruptcy Code. |
| 13.5. | Termination on a Country-by-Country Basis. In the event of the occurrence of a termination event or failure to cure under the terms of Section 13.2, 13.3 or 13.4, the non-breaching Party shall have the right to terminate this Agreement in its entirety; provided that if MGI is the terminating Party, it may elect to terminate this Agreement only in a given country in the Territory where such termination event relates to the Development or Commercialization of the Licensed Products in such given country. Any termination hereunder shall be by written notice of termination, which shall be effective on the date such notice is received pursuant to the provisions of Section 15.12. | ||
| 13.6. | Tolling of Cure Period. In the event of a dispute as to whether performance has been made by either Party pursuant to this Agreement, the relevant cure period with respect thereto shall be tolled pending resolution of such dispute in accordance with the applicable provisions of this Agreement. |
| 14.1. | Accrued Obligations. Termination or expiration of this Agreement for any reason shall not release any Party hereto from any liability (including payments due for a period prior to such termination or expiration) which, at the time of such termination |
54
| or expiration, has already accrued to the other Party or which is attributable to a period prior to such termination or expiration nor preclude either Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach of this Agreement. | |||
| 14.2. | Effects of any Expiration or Termination. Upon any expiration or termination of this Agreement, in whole or in part, Articles 7, 8, 10, 12, 14, 15, and Sections 5.11 and 9.4 shall survive with respect to the Licensed Products sold by or under authority of Licensee. |
| (a) | Licensee’s rights and licenses hereunder shall survive solely to the extent necessary for Licensee to perform its responsibilities pursuant to Section 14.3 below. Neither Licensee nor its Affiliates shall have any right to grant or authorize any rights or licenses under Section 2.1. All other terms and conditions shall terminate and have no further force or effect. For clarity, in the event that rights are terminated only with respect to certain countries in the Territory, the Development and Commercialization of such Licensed Products or in such countries by and under authority of MGI shall not be subject to Section 2.3. | ||
| (b) | In the event of termination of this Agreement under Section 13.2(b) or termination due to insolvency or material breach by Licensee, all rights of cross reference that have been provided to Licensee shall terminate, and all prosecution and enforcement of Licensed Patents by or under authority of Licensee shall be transitioned to MGI promptly, except to the extent otherwise agreed in writing. | ||
| (c) | In the event this Agreement is terminated in its entirety due to the material breach thereof by the Licensee, or if this Agreement is terminated by Licensee under Section 13.2(b) or 13.2(c), in addition to any other obligations set out in this Agreement, Licensee shall return to MGI, at Licensee’s cost and expense, all existing information, data, intellectual property rights, administrative authorizations and of all other know-how relating to the development and commercialization of the Licensed Products in Licensee’s possession, and ownership of the same shall revert to MGI. Termination by MGI for material breach by Licensee relating to any royalty payment owed to MGI hereunder will constitute a breach only with regard to the country(ies) in the Territory to which the breach specifically applies. Termination by MGI for material breach by Licensee relating to any development milestone payment owed to MGI hereunder shall constitute a breach of this Agreement in its entirety. |
| 14.3. | Rights on Expiration or Termination. References to termination in this Article 14 shall be deemed to include expiration of this Agreement. Upon any termination of this Agreement in its entirety, the following shall apply. In the event that this |
55
| Agreement is terminated only with respect to a country in the Territory, then the following shall apply. |
| (a) | Wind-down Period. Following expiration of this Agreement or termination as set forth above, the Parties agree as follows, provided that at its option, MGI may terminate the Wind-down Period as otherwise set forth herein, upon thirty (30) days written notice to Licensee. As used herein, “Wind-down Period” shall mean the one ninety (90) day period following the termination of this Agreement. | ||
| (b) | Development. In the event that the Licensed Products is under Development in the Territory, at MGI’s request, Licensee agrees to continue for a period not to exceed ninety (90) days after such termination the Development at its own expense, and to transition such Development to MGI to the extent requested. | ||
| (c) | Commercialization. At MGI’s request, if Marketing Authorization has been or is obtained for the Licensed Products, then Licensee and its Affiliates shall continue to Commercialize the Licensed Products in the same manner in each country in the Territory for which Marketing Authorization therefore has been obtained, in accordance with the terms and conditions of this Agreement, for a period not to exceed two hundred forty (240) days following such termination it being understood that MGI and Licensee will work diligently to transition such activities as soon as reasonably practicable. Notwithstanding any other provision of this Agreement, during the Wind-down Period, Licensee’s and its Affiliates’ rights with respect to the Licensed Products shall be non-exclusive, and MGI shall have the right to engage one or more other distributor(s) and/or licensee(s) to Develop and Commercialize the Licensed Products in all portions of the Territory that have been terminated. Article 3 shall continue to apply with respect to all the Licensed Products sold, used or disposed by Licensee and its Affiliates during the Wind-down Period or otherwise as set forth below. All rights of Licensee and its Affiliates with respect to the Licensed Products shall be terminated at the end of the Wind-down Period, and unless otherwise mutually agreed or unless rights have been terminated only with respect to a specified country, and Licensee retains rights to Develop and Commercialize the Licensed Products for other countries, all the Licensed Products in the possession of Licensee and its Affiliates that are not sold during such Wind-down Period shall be destroyed, provided, however, that Licensee shall have a minimum of one hundred-eighty (180) days following termination of the Wind-down Period to sell its remaining inventory, without regard to the actual length of the Wind-down Period. Any remaining unsold inventory shall be transferred to MGI at cost. | ||
| (d) | Assignment of MAA and Marketing Authorizations. Licensee shall assign or cause to be assigned to MGI (or if not feasible to immediately assign, |
56
| Licensee shall take all reasonable actions to make available to MGI (including by providing copies), and to give MGI and its Affiliates and its designees a right of reference to) all Regulatory Documentation (including XXXx and Marketing Authorizations) and Supporting Data for the Licensed Products obtained in Licensee’s name hereunder. In each case the foregoing assignment (or availability and right of reference) shall be made or provided no later fifteen (15) days after termination, and Licensee shall take, and shall cause its Affiliates to take, such actions and execute such other instruments, assignments and documents as may be necessary to effect the transfer of rights thereunder to MGI. In addition, Licensee shall promptly provide to MGI a copy of all Regulatory Documentation, Supporting Data, and Confidential Information pertaining to the Licensed Products to the extent not previously provided to MGI. MGI shall have sole control over all Regulatory Documentation and meetings and communications with Regulatory Authorities immediately upon termination. | |||
| (e) | Transition. Licensee shall use Diligent Efforts, as defined in Section 1.24, to cooperate with MGI and its designee(s) to effect a smooth and orderly transition in the Development and Commercialization of the Licensed Products in the Territory. Without limiting the foregoing, Licensee shall provide MGI copies of Sales Materials, Licensed Products labeling, other customer information, each relating to the Licensed Products to the extent not previously provided to MGI. In addition, Licensee shall refer all inquiries regarding the Licensed Products after the Wind-down Period (and during the Wind-down Period to the extent requested by MGI) to MGI and its designees. In addition, Licensee shall provide to MGI a list of all customers purchasing the Licensed Products from Licensee in the year prior to termination or expiration of the Agreement. |
| 14.4. | Rights. To the extent not assigned to or owned by MGI, Licensee hereby grants to MGI, effective following termination of this Agreement but no later than the end of the Wind-down Period, a worldwide, irrevocable, fully paid, royalty free, right and license, under all Sales Materials, and Confidential Information, with the right to grant and authorize sublicenses, to use, disclose, reference, publicly display, modify, reproduce, distribute, and otherwise exploit and dispose of the foregoing for any purpose, including the right to make, have made, use, sell, offer to sell, import and otherwise exploit and dispose of the Licensed Products in the Territory; provided that MGI shall not exercise the foregoing rights and licenses until termination of this Agreement in whole or in part. |
| (a) | Return of Materials. Within thirty (30) days after the end of the Wind-down Period, Licensee shall, and shall cause its Affiliates to, deliver to MGI, or to the extent requested by MGI destroy, all materials containing the Product Trademark, Supporting Data, and other Confidential Information of MGI, (except to the extent otherwise authorized by MGI) that is in Licensee’s or its |
57
| Affiliates’ possession or control, and provide written certification thereof. Effective upon the end of the Wind-down Period (or sooner if requested by MGI), Licensee shall cease, and shall cause its Affiliates to cease, all use of the Product Trademark, Regulatory Documentation, Supporting Data, and Confidential Information of MGI, with respect to terminated Licensed Products in the terminated countries in the Territory. | |||
| (b) | Rights on termination pursuant to Section 13.2(b) and (c). In the event of termination under Section 13.2(b) or (c) of this Agreement, Licensee will transfer information and Licensed Product consistent with the provisions of this Section 14, except that Licensee, at its option, may elect to cease all sales and marketing activities in one or more countries or in the Territory, at its sole discretion. |
| 15.1. | Article and Section Headings, Language and Construction. The Article and Section headings contained in this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement. All references in this Agreement to “Articles,” “Sections,” “Exhibits” and “Schedules” refer to the articles, sections and exhibits of this Agreement. The words “hereof,” “herein” and “hereunder” and other words of similar import refer to this Agreement as a whole and not to any subdivision contained in this Agreement. The words “include” and “including” when used herein are not exclusive and mean “include, without limitation” and “including, without limitation,” respectively. References in this Agreement to “days” shall mean calendar days unless expressly indicated to the contrary. This Agreement has been negotiated by the Parties and their respective counsel. Accordingly, this Agreement will be interpreted fairly in accordance with its terms and without any strict construction in favor of or against either Party. | ||
| 15.2. | Governing Law. Governing Law. This Agreement shall be governed in all respects by the internal laws of the State of New York as applied to contracts entered into solely between residents of, and to be performed entirely within, such state, and without reference to principles of conflicts of laws or choice of laws. | ||
| 15.3. | Submission to Jurisdiction. Each of the Parties irrevocably agrees that any legal action or proceeding with respect to this Agreement or for recognition and enforcement of any judgment in respect hereof brought by the other Party hereto or its successors or assigns shall be brought and determined in the federal courts located in the State of New York, and each of the Parties hereby irrevocably submits with regard to any such action or proceeding for itself and in respect to its property, generally and unconditionally, to the exclusive jurisdiction of the aforesaid courts. | ||
| 15.4. | Waiver of Jury Trial. EACH PARTY HEREBY IRREVOCABLY WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY ACTION, PROCEEDING OR |
58
| COUNTERCLAIM (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE ACTIONS OF THE PARTIES IN THE NEGOTIATION, ADMINISTRATION, PERFORMANCE AND ENFORCEMENT HEREOF. | |||
| 15.5. | Other Remedies. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a Party will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude the exercise of any other remedy. | ||
| 15.6. | Injunctive Relief. Because of, among other things, the unique nature of the Licensed Patents, Product Improvements and Licensed Know-How which are the subject of this Agreement, the potential that lost opportunity costs may not be quantifiable, the Parties hereto agree that irreparable damage may occur in the event that any of the provisions of this Agreement, were not performed in accordance with their specific terms or were otherwise breached. The Parties accordingly agree that injunctive relief enforcing the terms of this Agreement may be appropriate. | ||
| 15.7. | Dispute Resolution. Except as otherwise agreed, disputes arising within the JOC shall be referred to the Joint Steering Committee for resolution (“Dispute Resolution”). Disputes arising within the JOC which cannot be resolved by the Joint Steering Committee, disputes arising within the Joint Steering Committee, and any and all other disputes between the Parties arising out of or relating to this Agreement or the negotiation, performance, termination, validity or breach thereof, shall be resolved as follows and in the following order of preference: by good faith negotiation between the CEOs of MGI and Licensee, who shall have the authority to fully and finally resolve the dispute. In the event the designated Executive Officers are not able to resolve such dispute, either Party may elect within the following fourteen (14) calendar day period to resolve the dispute by conducting non-binding mediation; either Party may at any time following such mediation or after the expiry of the fourteen (14) calendar day period if the Parties do not elect to conduct non-binding mediation, seek to resolve the dispute through other means as provided herein. | ||
| 15.8. | Alternative Dispute Resolution. Any dispute controversy or claim arising out of or relating to the validity, construction, enforceability or performance of this Agreement, including disputes relating to an alleged breach or to termination of this Agreement, but excluding (a) any dispute, controversy or claim arising out of or relating to the validity, enforceability, or infringement of any Licensed Patent and (b) other than disputes which are expressly prohibited herein from being resolved by this mechanism, shall be settled by binding Alternative Dispute Resolution (“DR”) in the manner described below: |
| (a) | Notice of Arbitration. If a Party intends to begin a DR to resolve a dispute, such Party shall provide written notice (the “DR Request”) to counsel for the |
59
| other Party informing such other Party of such intention and the issues to be resolved. Within thirty (30) calendar days after receipt of the DR Request, the other Party may, by written notice to counsel for the Party initiating DR, add additional issues to be resolved. | |||
| (b) | Arbitration Procedures. A DR initiated under this Agreement will proceed in accordance with the Commercial Arbitration Rules of the American Arbitration Association for Large, Complex Cases then in effect, insofar as such rules are not inconsistent with the provisions expressly set forth in this Agreement, unless the Parties mutually agree otherwise. However, the Parties do not intend to submit the issue for resolution by such association unless they mutually agree to do so, in writing. | ||
| (c) | Arbitrators. The arbitration shall be carried out by a single arbitrator who shall be a retired United States judge or justice and shall be selected by the Parties within thirty (30) calendar days of receipt of the DR Request in accordance with the procedure described below: |
| (i.) | Qualifications. The Parties shall select the arbitrator as described below, which may but need not be selected from a list of arbitrators such as the CPR Panel of Distinguished Neutrals of the Center for Public Resources, subject to: (a) his/her availability and willingness to serve, (b) his/her availability to commence the arbitration within a reasonable period of time, (c) his/her agreement to charge fees and expenses that are reasonable under the circumstances, and (d) his/her commitment to render his/her award within the time periods provided in this Section 15.8. | ||
| (ii.) | Selection. Each Party will exchange a list of ten (10) qualified arbitrators and in the event that both Parties agree on a single person then such person shall be the arbitrator. In submitting the ten names, each Party shall prioritize from one to ten the persons on their respective lists. In the event that there is more than one common name on the Parties’ lists, the person having the lowest combined priority number shall be selected as the arbitrator. The combined priority number shall be the sum of the order numbers assigned to that person by the Parties. Thus, if one person was MGI’s number two priority and Licensee’s number three priority, and another person was MGI’s number two priority and Licensee’s number four priority, the former would be appointed. If more than one person has the lowest combined priority number, the person for whom there is less difference between the order numbers assigned by the Parties shall be appointed. Thus, if one person was MGI’s number one priority and Licensee’s number four priority, and another person was MGI’s number two priority and Licensee’s number three priority, the latter person would be appointed. If this method does not produce an arbitrator, or if there are no common names, the Parties shall alternatively strike from the combined list until only one name remains, which shall be |
60
| selected as the arbitrator. The Party to strike first shall be determined by the toss of a coin. In the event the arbitrator is unable to meet the requirements set forth in (i) above, then, in the event the first selected arbitrator was common to both lists and there was more than one common name on the Parties’ lists, the arbitrator having the next lowest combined priority number who is able and willing to serve pursuant to these requirements shall be selected. If there is no such individual, then the Parties shall use the alternate strike method set forth above. In the event an arbitrator selected by the alternate strike methodology is unable or unwilling to serve consistent with the requirements set forth above, then the alternate striking procedure shall be retraced in reverse order until the arbitrator is selected. | |||
| (iii.) | Conduct. The arbitrator shall be neutral, disinterested, impartial, and independent of the Parties and others having any known interest in the outcome, and shall abide by the AAA/ABA Code of Ethics for Arbitrators in Commercial Disputes. There shall be no ex parte communications with the arbitrator either before or during the arbitration, relating to the dispute or issues involved in the dispute or the arbitrator’s views on any such issue. | ||
| (iv.) | Interim Review. Either Party may apply to any court having jurisdiction hereof and seek preliminary injunctive relief until such time as the arbitration award is rendered or the controversy is otherwise resolved. | ||
| (v.) | Location. Any arbitration under this Section 15.8 shall be conducted in New York, NY. |
| (d) | Discovery Proceedings. The Parties shall have the right to undertake complete discovery, as contemplated by and pursuant to Federal Rules 26-37 of the Federal Rules of Civil Procedure (with references to “court” in those Rules being considered references to the “arbitrator”) except as they may be modified by the following: |
| (i.) | Within thirty (30) calendar days after selection of the arbitrator, each Party may serve on any other Party (a) interrogatories for the purpose of identification of documents and witnesses, (b) requests for admissions, and (c) requests for the production of documents and things. These discovery requests will be answered within fourteen (14) calendar days of receipt. | ||
| (ii.) | At any time but not more than six (6) months after the selection of the arbitrator, each Party may take depositions of employees or other witnesses of an opposing Party as a matter of right, subject to applicable limitations set forth in Rules 26-37. |
61
| (iii.) | Any Party may conduct depositions of its own witnesses which may be introduced as evidence at the arbitration hearing if the other Party was given fair opportunity to attend the deposition and cross-examine. | ||
| (iv.) | As they become aware of new documents or information, including experts who may be called upon to testify, all Parties remain under a continuing obligation to provide documents upon which they rely, to supplement their responses, and to honor any informal agreements or understandings between the Parties regarding documents or information to be exchanged. The arbitrator at the hearing will not consider documents that have not been previously exchanged, unless agreed by the Parties or used for purposes of impeachment. | ||
| (v.) | The Parties will promptly notify the arbitrator when an unresolved dispute exists regarding discovery issues. The arbitrator will discuss the matter with the Parties to determine the nature of the dispute and will attempt to resolve that dispute. If the arbitrator does not resolve the dispute, the arbitrators will arrange a conference concerning the dispute before the arbitrator by telephone, or in person, and the arbitrator will decide the dispute. | ||
| (vi.) | All discovery shall be completed within six (6) months from selection of the arbitrator, unless extended by agreement of the Parties or by the arbitrator for good cause shown. |
| (e) | Hearings and Proceedings. The arbitrator will determine the specific location within New York, NY and the date and time of the arbitration hearing(s) and other proceedings after consultation with the arbitrator and the Parties and will provide reasonable notice of the hearing(s) date and time. The arbitrator will make every effort to schedule the arbitration hearing within ninety (90) calendar days of the completion of all discovery, absent unusual circumstances. |
| (i.) | The Parties may mutually agree on or the arbitrator for good cause may order a rescheduling of the hearing date. | ||
| (ii.) | The arbitrator will ordinarily conduct the arbitration hearing in the manner set forth in this Section 15.8 except that (i) the Federal Rules of Evidence shall apply and (ii) the arbitrator shall render its decision in writing in the manner provided by Rule 52 of the Federal Rules of Civil Procedure, including a full and precise statement of the factual and legal bases for such decision. If the AAA rules and the rules of this Section 15.8 conflict in any manner, the rules of this Section shall prevail. The arbitrator must hold an oral hearing, but may impose reasonable time limits on each phase of the proceeding and may limit testimony to exclude evidence that would be immaterial or unduly repetitive, provided that all Parties are afforded the opportunity to present material and |
62
| relevant evidence and that each Party is given at least an approximately equal amount of time for presentation of its case. | |||
| (iii.) | Written briefs may be filed up to five (5) calendar days before the hearing. | ||
| (iv.) | The arbitrator will require witnesses to testify under oath if requested by any Party. | ||
| (v.) | Any Party desiring a stenographic record may secure a court reporter to attend the proceedings. | ||
| (vi.) | The arbitrator will determine the order of proof, which will generally be similar to that of a court trial, including opening and closing statements. | ||
| (vii.) | When the arbitrator determines that all relevant and material evidence and arguments have been presented, the arbitrator will declare the hearing closed. The arbitrator may defer the closing of the hearing for up to twenty (20) calendar days to permit the Parties to submit post-hearing briefs and or to make closing arguments, as the arbitrator deems appropriate, before rendering an award. | ||
| (viii.) | The arbitrator will render the award and its decisions pursuant to Fed. R. Civ. P. 52 within thirty (30) calendar days after the date of the closing of the hearing or, if an arbitration hearing has been waived, within thirty (30) calendar days after the date of the arbitrator’s receiving all materials specified by the Parties. The decision and award of the arbitrator will constitute the Arbitration Award and will be binding on the Parties. | ||
| (ix.) | The arbitrator shall, in rendering his or her decision and award, apply the substantive law of the State of New York, without regard to its conflict of laws provisions, except that the interpretation of and enforcement of this Section shall be governed by the Federal Arbitration Act. The costs of the winning Party and the fees of the arbitrator shall be paid by the losing Party as determined by the arbitrator. If the arbitrator is unable to designate a losing party, it shall so state and each Party shall bear its own costs and the fees of the arbitrator shall be split equally between the Parties. |
| (f) | Award. The arbitrator is empowered to award any remedy allowed by law, including money damages, prejudgment interest and attorneys’ fees, and to grant final, complete, interim, or interlocutory relief, including injunctive relief. Notwithstanding the foregoing, punitive or multiple damages may not be awarded. | ||
| (g) | Legal Fees. Except as set forth in Section 15.8(e)(ix) above, each Party shall bear its own legal fees. |
63
| (h) | Confidentiality. The DR proceeding shall be confidential and the arbitrator shall issue appropriate protective orders to safeguard each Party’s Confidential Information. Except as required by law, no Party shall make (or instruct the arbitrator to make) any public announcement with respect to the proceedings or decision of the arbitrator without prior written consent of each other Party. The existence of any dispute submitted to DR, and the award, shall be kept in confidence by the Parties and the arbitrator, except as required in connection with the enforcement of such award or as otherwise required by applicable law. | ||
| (i) | Survivability. Any duty to arbitrate under this Agreement shall remain in effect and enforceable after termination of this Agreement for any reason. |
| 15.9 | Jurisdiction. For the purposes of this Article 15 each Party agrees to abide by the award rendered in any arbitration, and the Parties agree to accept the jurisdiction of any court having jurisdiction over the Parties for the purposes of enforcing awards entered pursuant to this Article 15 and for enforcing the agreements reflected in this Article 15, provided that the foregoing shall not be deemed to waive any rights of either Party under the Federal Arbitration Act. | ||
| 15.10 | No Implied Waivers; Rights Cumulative. It is agreed that no waiver by either Party hereto of any breach or default of any of the covenants, representations, or warranties herein set forth shall be effective unless in a writing signed by such Party, and no such written waiver shall be deemed a waiver as to any subsequent and/or similar breach or default. | ||
| 15.11 | Independent Contractors. The relationship of Licensee and MGI established by this Agreement is that of independent contractors. Nothing in this Agreement shall be construed to create any relationship other than independent contractors. Licensee and MGI shall under no circumstances be considered engaged in a partnership or joint venture by virtue of this Agreement. Neither Party shall have any right, power or authority to assume, create or incur any expense, liability or obligation, express or implied, on behalf of the other or to conclude contracts in the name of the other. | ||
| 15.12 | Notices. Any notice required or permitted to be given hereunder shall be in writing and shall be delivered in person, by a nationally recognized overnight courier, or by facsimile (receipt confirmed, with a confirmation notice sent by one of the other methods specified herein), to the addresses given below or such other addresses as may be designated in writing by the Parties from time to time during the term, and shall be deemed to have been given when received unless otherwise specified herein as when sent. |
| Licensee: | CILAG GmbH INTERNATIONAL | |||
| Xxxxxx + Xxx-Xxxxxxx 0 | ||||
| XX-0000 | ||||
| Xxx, Xxxxxxxxxxx | ||||
| Attention: Company President |
| * | Denotes confidential information that has been omitted from the exhibit and filed separately, accompanied by a confidential treatment request, with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934. |
64
| Facsimile: 41 41 7255101 | ||||
| With copy to: | ||||
| Xxxxxxx & Xxxxxxx | ||||
| Xxx Xxxxxxx & Xxxxxxx Xxxxx | ||||
| Xxx Xxxxxxxxx, Xxx Xxxxxx 00000 | ||||
| Attention: Chief Patent Counsel | ||||
| Facsimile: 000- 000-0000 | ||||
| MGI: | ||||
| MGI PHARMA, INC. | ||||
| 0000 Xxxx Xxx Xxxxxxxx Xxxx, Xxxxx 000 | ||||
| Xxxxxxxxxxx, XX 00000-0000 | ||||
| Attn: Xxxx X. Xxxxxx, General Counsel | ||||
| Telephone: 000-000-0000 | ||||
| Facsimile: 000-000-0000 |
| or to such other address (including electronic mail address) as MGI shall have furnished to Licensee in writing or by electronic mail. Notices that are mailed by (i) first class mail shall be deemed received three (3) business days after deposit in the mail and (ii) Express Mail or overnight courier shall be deemed received one (1) business day after deposit in the mail or delivery to such courier. In the event that the notice is sent by facsimile, notice shall be deemed to have been received when sent and confirmed as to receipt. | |||
| 15.13 | Assignment. This Agreement shall not be assignable by Licensee to any other person or entity without the written consent of MGI, which shall not be unreasonably withheld; provided that either Party shall, without the consent of the other Party, be entitled to assign its rights to a third party of reasonably equivalent capabilities in the development and commercialization of pharmaceutical products, in the event of a merger or consolidation with, or the transfer of substantially all of its assets to such third party; provided, however, that should Licensee make such an assignment of this Agreement with a Third Party that is marketing or developing any Competing Product or that product competitive to the Licensed Products with the trade name Vidaza, in the Territory, and such Third Party does not take action to divest the same under Section 13.3(b)(ii), MGI may terminate this Agreement immediately and treat the termination as one due to a material breach of this Agreement by said Third Party. | ||
| 15.14 | Modification. No amendment or modification of any provision of this Agreement shall be effective unless in writing signed by all Parties hereto. No provision of this Agreement shall be varied, contradicted or explained by any oral agreement, course of dealing or performance or any other matter not set forth in an agreement in writing and signed by all Parties. |
65
| 15.15 | Severability. If any provision hereof should be held invalid, illegal or unenforceable in any jurisdiction, the Parties shall negotiate in good faith a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and all other provisions hereof shall remain in full force and effect in such jurisdiction and shall be liberally construed in order to carry out the intentions of the Parties hereto as nearly as may be possible. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction. | ||
| 15.16 | Publicity Review. Neither Party shall originate any written publicity, news release or other public announcement or statement relating to the announcement or terms of this Agreement (collectively, a “Public Disclosure”), without the prior review and written approval of the other Party, which approval shall not be unreasonably withheld or delayed. Notwithstanding the foregoing, either Party may make any Public Disclosure it believes in good faith based upon the advice of counsel is required by applicable law, rule or regulation or any listing or trading agreement concerning its or its Affiliates’ publicly traded securities; provided, however, that such Public Disclosure shall minimize to the extent possible the financial information disclosed, and that prior to making such Public Disclosure, the disclosing Party shall provide to the other Party a copy of the materials proposed to be disclosed and provide the receiving Party with an opportunity to promptly review the Public Disclosure. Notwithstanding the foregoing, the Parties agreed to a press release to announce the execution of this Agreement, attached hereto as Schedule 2.0; thereafter, Licensee and MGI may each disclose the information contained in such press release without the need for further approval by the other. In addition, notwithstanding anything to the contrary, each Party shall have the right to disclose the existence and terms of this Agreement to the extent set forth in Section 10.5 above. | ||
| 15.17 | Counterparts. This Agreement may be executed in two counterparts, each of which shall be deemed an original, and all of which together, shall constitute one and the same instrument. | ||
| 15.18 | Export Laws. Notwithstanding anything to the contrary contained herein, all obligations of MGI and Licensee are subject to prior compliance with United States and foreign export regulations and such other United States and foreign laws and regulations as may be applicable, and to obtaining all necessary approvals required by the applicable agencies of the governments of the United States and foreign jurisdictions. MGI and Licensee shall cooperate with each other and shall provide assistance to the other as reasonably necessary to obtain any required approvals. | ||
| 15.19 | Limitation of Liability. IN NO EVENT SHALL EITHER PARTY BE LIABLE TO THE OTHER FOR ANY INCIDENTAL, CONSEQUENTIAL, INDIRECT OR SPECIAL DAMAGES, HOWEVER CAUSED AND WHETHER BASED IN CONTRACT, TORT OR UNDER ANY OTHER THEORY OF LIABILITY. THE FOREGOING LIMITATIONS FURTHER SHALL NOT APPLY TO EITHER |
66
| PARTY’S OBLIGATIONS UNDER ARTICLE 12, OR EITHER PARTY’S BREACH OF ARTICLE 10. The Parties agree that nothing in this Section 15.19 shall in any way affect or impair the ability of the Parties to seek injunctive relief pursuant to Section 15.6 hereof. | |||
| 15.20 | Entire Agreement. This Agreement, together with all the Schedules hereto constitute the full and entire understanding and agreement between the Parties with respect to the subject matter hereof, and supersede all prior and contemporaneous agreements, understandings, negotiations and discussions, whether oral or written, of the Parties with respect thereto, including any term sheet. |
MGI PHARMA, INC.
|
LICENSEE: | |||||
/s/ Xxxx X. Xxxxxxx, Xx.
|
/s/ Xxxxx Xxxxxx | |||||
Title: President and CEO
|
Title: General Manager |
Schedule 1.7
|
J&J Universal Calendar – 2006 | |
Schedule 1.18
|
Decitabine | |
Schedule 1.51
|
Licensed Patents | |
Schedule 1.66
|
Orphan Product Designation for Decitabine in EC | |
Schedule 1.75
|
Manufacturing and Supply Agreements | |
Schedule 1.80
|
License Agreement between SuperGen, Inc. and MGI Pharma, Inc. | |
Schedule 1.90
|
R&D/GCP Audit Plan | |
Schedule 2.0
|
Press Release | |
Schedule 2.1
|
Example of Currency Conversion | |
Schedule 3.0
|
Initial Global Development Plan & Listing of On-going Clinical Trial | |
Schedule 6.1(b)
|
Minimum Product Order Quantities | |
Schedule 6.1(c)
|
Licensee Initial Product Forecast | |
Schedule 6.1(e)
|
Form of Purchase Order |
67
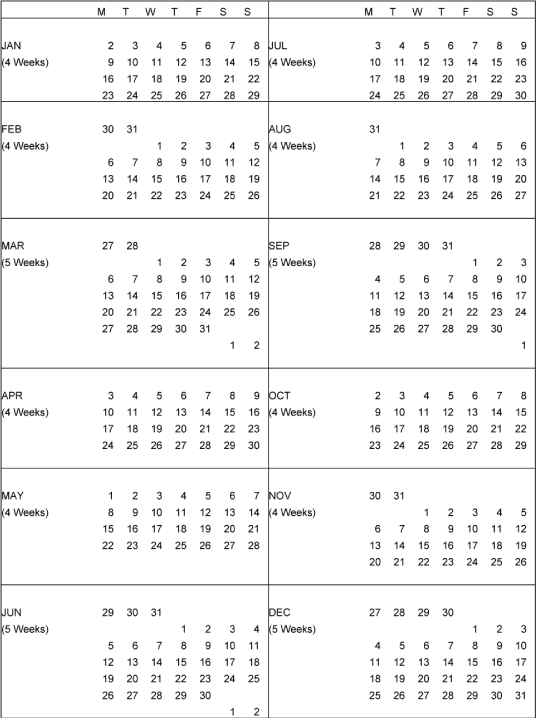
| US/Foreign Patent | ||||||
| Title | Number | Filing Date | Status | |||
* |
* | * | * | |||
* |
* | * | * | |||
RESTORING CANCER-SUPPRESSING FUNCTIONS TO NEOPLASTIC CELLS THROUGH DNA
HYPOMETHYLATION |
US10/686,047 (US2004-0109846) |
10/14/2003 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
RESTORE CANCER-SUPPRESSING FUNCTIONS TO NEOPLASTIC CELLS THROUGH DNA HYPOMETHYLATION |
EP 02717415.0 (EP 1383379) |
2/11/2002 | Published | |||
RESTORE CANCER-SUPPRESSING FUNCTIONS TO NEOPLASTIC CELLS THROUGH DNA HYPOMETHYLATION |
JP 2002-567063 (JP 04-529104) |
2/11/2002 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
LIQUID FORMULATION OF DECITABINE AND USE OF THE SAME |
EP03757313.6 (EP1509233) | 5/30/2003 | Published | |||
LIQUID FORMULATION OF DECITABINE AND USE OF THE SAME |
JP2004-510806 (JP05-529163) | 5/30/2003 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
METHOD FOR TREATING DISEASES ASSOCIATED WITH ABNORMAL KINASE ACTIVITY |
EP03710881.2 (EP1572075) | 2/6/2003 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
| US/Foreign Patent | ||||||
| Title | Number | Filing Date | Status | |||
COMPOSITIONS AND METHODS FOR RE-ESTABLISHING GENE TRANSCRIPTION THROUGH INHIBITION
OF DNA METHYLATION AND HISTONE DEACETYLASE |
EP02731396.4 (EP1389127) | 4/19/2002 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
PHARMACEUTICAL FORMULATIONS TARGETING SPECIFIC REGIONS OF THE GASTROINTESTINAL TRACT |
US10/698,983 (US2004-0162263) |
10/31/2003 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
PHARMACEUTICAL FORMULATIONS TARGETING SPECIFIC REGIONS OF THE GASTROINTESTINAL TRACT |
EP03778052.5(EP1556010) | 10/31/2003 | Published | |||
COMPOSITIONS AND METHODS FOR TREATMENT OF CANCER |
US10/661,386 (US2005-0059682) |
9/12/2003 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
COMPOSITION AND METHOD FOR TREATING NEUROLOGICAL DISORDERS |
US10/877,046 (US2005-0037992) |
6/24/2004 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
COMPOSITIONS AND FORMULATIONS OF DECITABINE POLYMORPHS AND METHODS OF USE THEREOF |
US10/890,828 (US2006-0014949) |
7/13/2004 | Published | |||
COMPOSITIONS AND FORMULATIONS OF DECITABINE POLYMORPHS AND METHODS OF USE THEREOF |
PCT/US2005/24676 (WO 06/017278) | 7/12/2004 | Published | |||
* |
* | * | * | |||
ORAL ADMINISTRATION OF DECITABINE SALT |
11/238,168 (US2006-0074046) |
09/27/05 | Published | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * | |||
* |
* | * | * |
| 1. | Drug Substance and Validation and Supply Agreement between SuperGen, Inc. and Ferro Pfanstiehl Laboratories, Inc. and its affiliates, effective as of July 2, 2003 and Amendments 1, 2, 3, 4, 5, and 6 thereto |
| 3. | Manufacturing Agreement between MGI Pharma Inc. and Pharmachemie B.V., effective as of July 6, 2005 |
| 4. | Quality agreement between MGI Pharma Inc. and Pharmachemie B.V., effective September 8, 2005 |
Amended and Restated License Agreement between SuperGen, Inc. and MGI Pharma, Inc. effective as of
September 21, 2004 and amended as of July 3, 2006,
R&D/GCP Audit Plan
| * | * | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
| * | * | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
* |
* | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||||||||||||||||||||||||
*
*
*
Press Release
Initial Global Development Plan & Listing of On-going Clinical Trials

