SUPPLY AND DISTRIBUTION AGREEMENT #06-OEM-VC-EV-JLB
#06-OEM-VC-EV-JLB
2/39
| 2.1 | Product definition |
| a) | The VC s5i System complying with following specifications: |
| • | Primary CPU assembly with all acquisition, processing and I/O boards | ||
| • | VH circuitry and algorithms integrated with Primary CPU Assembly | ||
| • | Mounting bracket | ||
| • | LCD color Monitor | ||
| • | Data entry devices: Keyboard, track ball and point & click interfaces and roll-up carts if necessary. | ||
| • | Input power from 100 to 240 VAC, 50/60 Hz |
3/39
| • | ECG trigger card | ||
| • | Digital frame grabber | ||
| • | DVD Drive | ||
| • | Software package including Patient Entry, multiple image screen formats (Ultrasound, stored and sagittal views), Multiple Images Diameters, Automatic and manual measurements, In line Digital, Automatic Border Detection, Nine Minute Digital Video Storage, ChromaFlo, Lesion assessment package, DICOM 3.0 Services, and Virtual Histology Package (herein after referred to as “VH”) | ||
| • | Software package does not include 2D and/or 3D angio “QCA” or QCA-like software packages (such as Paieon / IVUS software packages). GEHC may wish to offer such software features to its End Users, prices and other commercial terms shall be negotiated separately between the parties, as appropriate. | ||
| • | Annual software maintenance agreements (to the exception of SW issues covered under the Article 14 and 11) are not included in the Price of the Products. If GEHC wishes to offer such software maintenance agreements to its End Users, prices and other commercial shall be negotiated separately between the parties, as appropriate. | ||
| • | Installation and Service Manuals in English | ||
| • | Packaging for Air / Road transportation | ||
| • | All “Special” installation tools and fixtures. | ||
| • | Regulatory approvals per Section 2.1 c) | ||
| • | Languages for User Manuals Graphical User Interface messages, Safety related messages and labels per Section 2.1 c) |
| b) | The VC s5i/GE System based on the VC s5i as defined in Section 2.1 a) and customized for GEHC (herein after referred to as “Customization”), including but not be limited to optimized integration of the s5i with the GEHC Cardiovascular system as further defined in Attachment A. | |
| In order to interface the VC s5i/GE System with the GEHC Cardiovascular system, a specific SW User Interface will be loaded on GEHC Touchscreen-type integrated cath lab control station Trademarked by GEHC as “Innova Central”) as further defined in Attachment A. | ||
| The IVUS connectivity feature on Innova will be branded by GEHC as “Innova IVUS”. Where applicable, a tag line “Powered by Volcano” shall be added to clarify that IVUS imaging will be performed by Volcano. The Innova IVUS feature shall not be considered as part of the s5i/GE supplied by VC. | ||
| c) | Regulatory Approvals, Manuals and languages |
| (i) | Prior to Product Introduction, VC shall obtain all applicable regulatory approvals and clearance for the Products to be distributed by GEHC in the US and all countries of the European Union, and provide the Products to GEHC including the corresponding labeling in the appropriate languages: |
4/39
| • | CE Marking for European Union | ||
| • | US FDA Approval | ||
| • | UL | ||
| • | WEEE compliant | ||
| • | Accompanying document for the Products must include Operator Manuals translated in accordance with the EU language policy of Attachment N (English, Spanish, French, German, Portuguese, Italian, ...) | ||
| • | Accompanying document for the Products must include Installation and Service Manuals in English | ||
| • | All Graphical User Interface messages, Safety related messages and labels translated in accordance with the EU language policy of Attachment N (English, Spanish, French, German, Portuguese, Italian, ...) |
| (ii) | Prior to Product Introduction, VC shall use commercially reasonable efforts to obtain all applicable regulatory approvals and clearance for the Products to be distributed by GEHC in the People Republic of China, and provide the Products to GEHC including the corresponding labeling in Chinese: |
| • | CCC Approval | ||
| • | SFDA Approval | ||
| • | System level name and rating plate in Chinese, warning labels in Chinese and CCC ▇▇▇▇ shall be applied to the Products before shipping. | ||
| • | Accompanying document for the Products must include Operator Manuals in Chinese | ||
| • | Accompanying document for the Products must include Installation and Service Manuals in English |
| (iii) | As soon as practical, VC shall make its best efforts to obtain approvals and clearance to distribute the s5i in all other major countries in the Territory such as China, major Asian countries, ANZ, Canada and Latin America (i.e. CCC & SFDA for China, CSA & CDMAS for Canada). When VC obtains such regulatory approvals and clearance for its s5i system, VC shall immediately make them available to GEHC for both the s5i and s5i/GE systems, including Graphical User interface messages, Safety related messages, labels and User Manuals, translated in the appropriate language. | ||
| (iv) | If GEHC identifies a business opportunity in a market or country not initially considered by VC, the Parties should discuss and agree if VC should proceed with the regulatory approval process and how VC for its agents, representatives or distributors) will provide GEHC End Users with application training and disposables. | ||
| (v) | When VC introduces improvements or new features to the s5i (such as an evolution of the s5i platform to support mechanically rotating transducer design), VC shall immediately make such improvements available to GEHC for both the s5i and s5i/GE systems. VC selling price to GEHC for any improvements or new features (additional options) to the s5i will be determined by mutual agreement between |
5/39
| the parties at the time of availability of such improvement or new feature. | |||
| (vi) | GEHC will obtain the appropriate regulatory approvals for the “Innova IVUS” as defined in Section 2.1 b). |
| (i) | VC and GEHC shall define and pursue jointly agreed integration and development objectives as relates to the s5i/GE in order to optimize the clinical efficacy and workflow combined technologies. VC and GE HC shall [CONFIDENTIAL] their efforts on system integration and development. | ||
| (ii) | VC will define, design, specify, select components, sub-assemblies, equipments, integrate and qualify them into a system, manufacture, test, procure the appropriate regulatory approvals and supply the Products to GEHC hereunder and as per the terms of this Agreement. VC will run the engineering development programs under its quality and regulatory framework for s5i and s5i/GE with inputs and reviews from GEHC side. VC will provide the required information to GEHC for the purpose of GEHC complying with its internal Product development / introduction processes (Milestones per GEHC’s Phase Review Discipline) and complying with applicable laws and regulations in relation with the Products and any parts thereof. | ||
| (iii) | The Parties agree that VC shall comply with the following development milestones: |
| Date | Task | |||
[CONFIDENTIAL] |
[CONFIDENTIAL] |
|||
6/39
| Date | Task | |||
[CONFIDENTIAL] |
[CONFIDENTIAL] |
|||
7/39
| Date | Task | |||
[CONFIDENTIAL] |
[CONFIDENTIAL] |
|||
| (iv) | GEHC and VC will jointly define the required performances for the Products to meet end user expectations and tenders requirements and shall agree on a set of minimum achievable specifications that will be translated into a contractual “Purchase Specification” and a contractual “Factory Acceptance Test” at the latest prior to the Delivery of the first Pilot. | ||
| (v) | VC agrees at all times to offer GEHC a competitive Product with regards to other existing systems available on the market. |
| 2.3 | Purchase and Distribution of Products. |
| (i) | During the Term of this Agreement and subject to Article 3, GEHC will purchase from VC, and VC will sell to GEHC, the Products, and GEHC will distribute the Products under VC trademarks, subject to the terms of this Agreement. Subject to Article 3, VC shall be required to supply the s5i/GE to GEHC exclusively, and to supply the s5i to GEHC non-exclusively. Except for the 8 pilots as further described in Article 5, GEHC’ commitment to purchase Products from VC shall be limited to firm Purchase Orders released by GEHC and accepted by VC pursuant to Article 8. | ||
| (ii) | Distribution will be performed by GEHC directly or through other members of the GEHC Group. |
| 2.4 | Representatives. GEHC may appoint third parties, including but not limited to parties that may be members of the GEHC Group, and such as agents, distributors, brokers to distribute, represent or promote the Products to End Users (each a “Representative”). VC currently maintains exclusive distribution agreements in select countries of the territory as outlined in Attachment M, but is actively engaged in modifying said existing agreements. GEHC’s ability to appoint third parties is dependent upon VC’s ability to modify its existing agreements. In priority, VC shall apply all its commercially reasonable efforts to modify its existing agreements and give access to GEHC in the following countries: Australia, Spain, Italy, China, Finland, Canada and New Zealand. If VC is not able to give GEHC distribution access to one of these country, the Parties shall agree on the new minimum quantity for GEHC to maintain its Exclusivity and distribution rights per Section 2.4, 3.2 and 23.3 (iii). The new minimum quantities shall reduced according to GEHC missed market opportunity in that country. |
8/39
| 2.5 | Independent Contractors. The relationship of GEHC and VC established by this Agreement is that of independent contractors, and nothing herein will be construed to (a) give either Party the power to direct or control the day-to-day activities of the other, (b) constitute the Parties as partners, joint venturers, principal and agent, employer and employee, co-owners, franchisor and franchisee, or otherwise as participants in a joint undertaking, or (c) allow either Party to create or assume any obligation on behalf of the other Party for any purpose whatsoever. Except as otherwise set forth herein, all financial and other obligations associated with each Party’s business are the sole responsibility of such Party. |
| 3.1 | Subject to Section 3.8, during the Term of this Agreement and subject to the Conditions below, VC will grant non-exclusive distribution rights to GEHC for the s5i System in the Territory. | |
| 3.2 | So long as GE forecasts to purchase a minimum of [CONFIDENTIAL] units of the s5i/GE during the first 12 months post Product Introduction (the “Exclusivity Period”), VC will grant exclusive distribution rights to GEHC for the s5i/GE System in the Territory during that 12 month Exclusivity Period. | |
| During this Exclusivity Period, VC will not sell, lease, loan, license, transfer or otherwise provide, or grant any distribution, or other rights to provide, directly or indirectly, the s5i/GE to any manufacturer, distributor, customer or other third party in the Territory, and will not pursue similar implementations as for the s5i / GE defined herein with any of the GEHC competitors in the Territory. | ||
| The Exclusivity Period shall start at the earlier of August 15, 2006 or Product Introduction, provided however that a delay in Product Introduction is not solely caused by VC, in which case the Exclusivity Period start date may be extended by 6 weeks, or longer if mutually agreed by the parties. During the Exclusivity Period, GEHC will not pursue similar implementations with any of the VC’s competitors. | ||
| Subject to Section 3.8, after the Exclusivity Period and during the Term of the Agreement, GEHC shall retain the rights to distribute the s5i/GE on a non-Exclusive basis in the Territory. | ||
| The Parties may consider extending the Exclusivity Period in exchange for certain minimum volume purchase commitments from GEHC. Similar implementations with non-competing third parties may be pursued with the reasonable consent of both parties. | ||
| 3.3 | Japan distribution rights for the 5i and s5i/GE will be subject to separate negotiation and agreement, should parties desire to pursue. |
9/39
| 3.4 | Exclusivity and distribution rights shall continue in the event of any change of control of VC ownership. | |
| 3.5 | VC acknowledges that at the time of signature of this Agreement, and subject to exceptions identified in Attachment M, it does not have, shall not maintain and shall stop any contractual obligations with third parties which contractually put VC in breach of its obligations in this Agreement, or contractual obligations which refrain VC from entering into this Agreement and from appointing GEHC as an exclusive distributor for the s5i/GE and as a non-exclusive distributor for the s5i in the Territory as per the terms herein. | |
| 3.6 | VC agrees that it will not, without the prior written consent of GEHC in each instance: use the name, trade name, trademark, trade device, service ▇▇▇▇, logo, symbol or any abbreviation, contraction or simulation thereof, owned by GEHC or by companies of the General Electric group, in any advertising, marketing, promotional materials, publicity, client list, press release, case studies, references, Internet posting, or otherwise. GEHC will consult VC prior to releasing advertising, marketing, promotional materials or issuing a press release all using the name, trade name, trademark, trade device, service ▇▇▇▇, logo, symbol or any abbreviation, contraction or simulation thereof, owned by VC. | |
| 3.7 | During the Exclusivity Period a defined in Section 3.2, GEHC will not distribute a competing product to the s5i/GE (IVUS integrated with a GEHC Innova System). Subject to Section 23.3 (iii), GEHC obligation not to distribute a competing product to the s5i/GE (IVUS integrated with a GEHC Innova System) will not apply in the event (i) where a company of GEHC Group acquires a company or part thereof already offering such a product or when (ii) GEHC exceptionally resells a competing product as requested by one of its End User. In the event GEHC Group acquires a company or part thereof already offering such a product, the Exclusivity Period shall terminate immediately. | |
| 3.8 | VC shall retain the right to market and distribute products similar to the s5i in all the countries and territories in the world. Notwithstanding anything to the contrary herein contained, VC shall not have the right to distribute products in the Territory containing key features or attributes considered as part of the Product’s Customization for GEHC, specifically optimized integration of the s5i with the GEHC Cardiovascular system and the SW User IF on a GEHC Touchscreen-type integrated cath lab control station Trademarked by GEHC as “Innova Central”), GEHC trade name, trademark, logo, color theme, user interfaces. |
| 4.1. | VC responsibilities | |
| Throughout the duration of this Agreement, and notwithstanding anything herein contained, VC shall be responsible and bear the corresponding costs for: |
10/39
| • | Defining, Specifying, Designing or Selecting components, integrating, qualifying them into a system, Manufacturing, Validating, Verifying, testing and packing the s5i and the s5i/GE for GEHC | ||
| • | Developing s5i and s5i/GE under VC’s quality and regulatory framework with appropriate input from GEHC | ||
| • | Supporting GEHC in the integration of the s5i/GE and s5i with the GEHC Cardiovascular systems | ||
| • | Subject to Section 2.1 c), obtaining all the appropriate regulatory approvals for GEHC to sell the s5i and the s5i/GE in all major markets of the world and lesser markets where practicable (excluding Japan) | ||
| • | Supplying the s5i and the s5i/GE to GEHC | ||
| • | Subject to Section 2.1 c), obtaining and maintaining the required Product certifications for GEHC to distribute them in the Territory, i.e. including but not limited to, USA, Europe, Canada, Latin America, China, e.g.: |
| o CE Marking | |||
| o US FDA Approval | |||
| o UL/CSA | |||
| o CMDCAS (Canada) | |||
| o SDA (China) | |||
| o CCC(China) |
| • | Developing unpacking, installation, preventive maintenance, troubleshooting and service procedures, spare parts lists and providing the associated documentation with each Product and part and sub-assemblies, equipment thereof, such as but not limited to Pre installation Manual, Service Manuals in the English language. | ||
| • | Subject to Section 2.1 c), Developing, translating and implementing End user interfaces messages | ||
| • | Subject to Section 2.1 c), developing, translating and providing the End-Users Operator Manuals with each Product in the following languages: |
| o English | |||
| o German | |||
| o French | |||
| o Spanish | |||
| o Portuguese | |||
| o Italian | |||
| o Chinese |
| • | Performing the end user application training for the s5i and the s5i/GE sold by GEHC within reason and per standard industry practice | ||
| • | Defining and providing GEHC personnel with the appropriate Installation and Service Training (Train the Trainer) in accordance with Article 10. | ||
| • | Assisting GEHC in collecting feedbacks from End-Users and service personnel upon installation of the Pilots installed and implementing corrective / improvement actions on subsequent Products to be delivered. | ||
| • | Providing information to GEHC for the purpose of GEHC’ complying with its internal Product development / introduction processes and complying with applicable laws and regulations in relation with the Products and any parts thereof. | ||
| • | Providing information and supporting GEHC in the commercial promotion of the Products in the Territory. |
11/39
| • | Throughout the duration of the Agreement and for a period of 10 years following the last manufacturing date of the Product, performing the second level of technical support to GEHC and handling, solving and closing field complaints related to the Products: |
| o PQRs: Significant quality or technical issue | |||
| o CSOs: Customer Satisfaction issues | |||
| o PSRs: Safety issues |
| • | Throughout the duration of the Agreement providing GEHC with Products spare parts for 10 years following the last manufacturing date of the Product in accordance with Article 11. | ||
| • | Throughout the duration of the Agreement and for a period of 10 years following the last manufacturing date of the Product and in case of product recall, safety, regulatory or severe performance issue, VC shall be responsible for defining, designing, manufacturing and supplying to GEHC Field Modification Instructions kits (“FMI”) for the purpose of remedying to such issues. VC shall supply such upgrade kits (including but not limited to Hardware, Software, instructions, packaging, tooling, ....) at no charge to GEHC for deployment on the impacted Products and participate to 50% of GEHC on-site direct labor cost (based on USD 93 / hour and the standard intervention time defined per the Field Modification Instruction, excluding travel and living that shall be bore by GEHC) | ||
| • | Supplying the Products and spare parts ordered by GEHC in the quantities and at the times specified by GEHC in its Purchase Orders in accordance with the terms and conditions of its Purchase Orders and this Agreement, assuming that GEHC’s order is within its forecasted range as outlined in Article 6 |
| 4.2. | GEHC responsibilities | |
| Throughout the duration of this Agreement, and notwithstanding anything herein contained, GEHC shall be responsible and bear the corresponding costs for: |
| • | Providing specified assistance to VC during the initial development phase of the Product with a view that the Product receives broad market acceptance and meet End-User expectations and support VC with the integration of the s5i/GE with the GEHC Cardiovascular systems | ||
| • | Providing VC with some specified training on certain GEHC’ applicable processes (Sourcing, Service, Support, etc) | ||
| • | Participating in design reviews specifically of s5i/GE as and when requested by VC | ||
| • | Offer the s5i and the s5i/GE as part of its Cardiovascular and Interventional Radiology product lines | ||
| • | Laying out system processes such that the s5i or s5i/GE are included as an option directly on all initial Cardiovascular and Interventional Radiology systems quotes after Product Introduction and provided regulatory approvals are in place. | ||
| • | Shipping and Installing and Servicing the Products sold by GEHC in accordance with VC’s instructions. | ||
| • | Coordinate application training with VC personnel for the s5i and s5i/GE systems sold by GEHC |
12/39
| • | Offer ongoing service under a GEHC service contract for the s5i and s5i/GE systems sold by GEHC. | ||
| • | Providing its expertise and support to VC in relation with certification in countries requiring a local representative (FDA 510K, MHW, SDA). | ||
| • | Obtaining the appropriate regulatory approvals for the “Innova IVUS” SW feature as defined in Section 2.1 b). | ||
| • | Loading the “Innova IVUS” SW feature onto the CardioVascular systems to be interfaced with the s5i/GE. | ||
| • | Providing support to VC in its negotiation with its key suppliers for the purpose of reducing costs and selling Prices to GEHC as well as improving suppliers lead-times and payment terms. | ||
| • | Validating the Service and Operator Manuals satisfactorily drafted by VC. | ||
| • | Collecting End-User and service feedbacks from the Pilot sites and communicating them to VC. | ||
| • | Defining and developing collaterals to promote the Products, including brochures, datasheets, trade shows. | ||
| • | Providing training to its own GEHC’ designated Sale, Service personnel subject to Article 10 | ||
| • | Communicating to VC all field complaints related to the Products and allowing VC to have direct access to GEHC customers, personal or representatives for the sole purpose of solving such complaints. | ||
| • | Regularly providing VC with market feedbacks on Products specifications and performances for the purpose of improving the Products competitiveness. |
| 5.1 | Subject to the term of this Agreement including Product Definition Section 2.1 and pursuant to the Purchase Orders for Pilots attached in Attachment J, GEHC is willing to purchase and take delivery from VC of a total quantity of 8 pilot “Products”, (collectively referenced herein as “Pilots”). The period starting from delivery of the first Pilot to the delivery of the last one is defined as the “Pilot Phase”. | |
| It is agreed that certain features of the Products or certain components of the software package, including but not limited to Virtual Histology, may not be available at Pilot phase or at Product Introduction as further described in Section 2.2. The parties agree that Pilots that are sold prior to fully meeting the Product Definition per Section 2.1, will be upgraded by Volcano as soon as practicable, at no cost to GEHC or the end-user. | ||
| 5.2 | The purpose of GEHC installing the Pilots at End-Users sites will be to ensure that: |
| • | The Products meet or exceed End-User expectations, | ||
| • | The Products can be easily installed and serviced by GEHC, | ||
| • | The GEHC Service, GEHC On Line Centers personal are satisfactorily trained, | ||
| • | The Product Service documentation and Operator documents are available and of good quality. |
13/39
| • | The processes between GEHC and VC are in place before entering in full production (Logistics — shipping, PQRs — CSOs — PSRs handling, spare parts ordering). |
| 5.3 | The VC selling price to GEHC for the 8 above-mentioned Pilots is subject the conditions of Article 7. All invoices will be settled according to the terms defined in Article 13. For these 8 pilots, VC should provide upgrades kits to Full Production level (as defined in section 2.1) at no cost to GEHC, GEHC covering the logistics and labor costs. | |
| 5.4 | Subject to the final approval of milestones identified in Article 2.2(iii), these 8 Pilots should start with 4 external evaluation units as soon as possible to be delivered by VC not later than May 1, 2006. These 4 external evaluation Pilots shall be installed and supported jointly by VC and GEHC engineering group, until such Pilots have been updated to the level of production units. GEHC shall have, at its discretion, the ability to order any combination of s5i and s5i/GE Products for the Pilot Phase such that the total of units ordered does not exceed 8 units. VC agrees to upgrade any Pilot units, at GEHC’s discretion, to s5i/GE units at no cost to GEHC, with GEHC covering the logistics and labor costs. | |
| In addition to the above-mentioned quantity, the number of Pilots may be extended by a mutually defined amount to seek broader customer feedback from varying market segments of interventional radiology and interventional cardiology. For such additional Pilots, VC shall provide upgrades kits to Full Production level (as defined in Section 2.1) at no cost to GEHC, GEHC covering the logistics and labor costs. | ||
| 5.5 | Notwithstanding anything to the contrary herein contained, GEHC will not be obliged to take Delivery of any Pilots or Products unless: |
| • | The Product is in full compliance with the Purchase Specification and passes the Factory Acceptance Test as defined in Section 2.2 (iii), and | ||
| • | For any Product to be sold in Europe, the Product is CE Marked approved, and | ||
| • | For any Product to be sold in the US, the Product is UL and FDA 510K approved, and | ||
| • | For any Product to be sold in China, the Product is CCC and, SDA approved, and | ||
| • | For any Product to be sold in Canada, the Product is CSA and CMDCAS approved, and | ||
| • | The Product is not under a recall as reported to a national authority. |
14/39
| 6.1 | The Parties agree that this Agreement will enter into the “Production Phase”, when GEHC estimates that sufficient feedbacks have been collected during the Pilot Phase, that the Product meets End User expectations, that all risks identified are retired, and that all issues are fixed and processes stabilized. GEHC and VC shall therefore agree in writing on an updated and fixed Purchase Specification and to a Factory Acceptance Test in order to reflect the required changes identified during the Pilot Phase. The Parties will work to finalize such Purchase Specification and Factory Acceptance Test at the latest 30 days after the Delivery of the last Pilot. Should the Parties fail to agree on the same by this date, GEHC will have the right to terminate this Agreement with a thirty days prior written notice, without any liability whatsoever. After agreement on the Purchase Specification and Factory Acceptance Test, such documents will be considered as Attachment B and an integral part of this Agreement and GEHC will then release forecasts and Purchase Orders to VC subject to the following provisions: | |
| 6.2. | Estimated volume. Subject to Section 6.3 below, GEHC’ anticipated volume at the signature date of this Agreement is estimated to be [CONFIDENTIAL] to [CONFIDENTIAL] Products during year 2006, and [CONFIDENTIAL] to [CONFIDENTIAL] units during year 2007. Despite the indicative nature of such estimate, VC represents and warrants herein that it has the capacity and expertise necessary to manufacture and deliver to GEHC the presently estimated volume of Products, and any increase up to 20 percent of such volume. | |
| 6.3. | Forecast. Notwithstanding anything to the contrary herein contained including Section 6.5, any forecast of GEHC’ requirements for Products shall not be binding in any way on GEHC. Any such forecast may be modified at any time by GEHC in its sole discretion. | |
| 6.4. | Commitment. During the production phase, GEHC’ commitment to purchase Products from VC shall be limited to firm Purchase Orders released by GEHC and accepted by VC pursuant to Article 8. Unless agreed otherwise in writing by the Parties, VC will not manufacture or assemble any Products, nor procure required materials except pursuant to accepted Purchase Orders or the provisions of Section 6.5. Unless agreed otherwise in writing by the Parties, GEHC shall not be responsible or in any way liable to VC or any third party with respect to any material commitments or production arrangements in excess of the amounts defined in Section 6.5. | |
| 6.5. | GEHC forecasts and orders to VC will consist of: |
| • | “Medium Term” forecasts, updated twice a year (Week 10 and Week 37) covering the second half of the current year and the first half of the following year | ||
| • | “Short Term” forecasts updated monthly and giving visibility of the current Quarter and the next Quarter. |
15/39
| • | Individual Purchase Orders to be communicated by GEHC to VC at least two weeks prior to Delivery. GEHC shall use its best efforts to communicate the Individual Purchase Orders as soon as reasonably possible, to allow VC to better plan its production. |
| 6.6. | Any failure by VC to deliver the Purchase Orders in accordance with the dates set forth in GEHC Purchase Orders and acknowledged by VC in accordance with Section 8.2, will subject VC to the provisions set forth in Section 15.3. |
| 7.1 | Pricing. VC selling price to GEHC for the s5i and s5i/GE Products shall be Ex-works Rancho Cordova, CA, USA (the “Prices’’). Prices shall include all packaging, date of manufacture and bar code labeling which GEHC may request from time to time. In no event shall the Prices be increased by VC throughout the duration of this Agreement. | |
| All prices referred to in this Agreement will be quoted in USD unless agreed otherwise in writing by the Parties. | ||
| Prices shall correspond to configurations of the s5i and s5i/GE allowing basic operation of the systems without the need for additional accessories. The Product shall consist of elements as defined in section 2.1. Selling prices for additional accessory parts (for more elaborate configurations) shall be agreed upon prior to Product Introduction. | ||
| Per the schedule below, the Prices shall be based on the actual volume of s5i and s5i/GE units (taken together) purchased by GEHC during a Calendar Year: |
| Number of Products | Price per unit | |||
| s5i and s5i/GE units taken together | s5i/GE or s5i | |||
| purchased by GEHC during a Calendar Year. | ||||
| [CONFIDENTIAL] to [CONFIDENTIAL] units | $ [CONFIDENTIAL] |
16/39
| [CONFIDENTIAL] to [CONFIDENTIAL] units | $ [CONFIDENTIAL] | |||
| [CONFIDENTIAL] units and more | $ [CONFIDENTIAL] |
| 7.2 | Mid Year and Year End Reconciliation. At the beginning of the Year, GEHC may decide to purchase the Products at a pre-discounted price (ie. $[CONFIDENTIAL] or $[CONFIDENTIAL] per unit). | |
| At the end of the calendar year, and based on the actual volume of Products purchased by GEHC either a balance rebate will be issued by VC to GEHC or a refund will be issued by GEHC to VC. | ||
| In order to minimize the Year End balance amount due by one Party to the other Party, the Parties may decide to perform a Mid Year Reconciliation and adjust the prices for the remaining of the calendar year accordingly. | ||
| For the remaining of the calendar year following the Product Introduction, minimum quantities required for GEHC to be qualified for discounted Price shall be Prorata temporis to the remaining of the calendar year. For the avoidance of doubt, if Product Introduction occurs on April 30th, 2006, the price of $[CONFIDENTIAL] / unit should be effective if GEHC orders at least 2/3 of [CONFIDENTIAL] units = [CONFIDENTIAL] Product during the remaining of 2006, and the Price of $ [CONFIDENTIAL] / unit should be effective if GEHC orders at least 2/3 of [CONFIDENTIAL] units = [CONFIDENTIAL] Product during the remaining of 2006. External Evaluation units and Pilots ordered by GEHC, whether shipped before or after the Product Introduction shall be included in the overall GEHC volume. | ||
| 7.3 | Image Co-Registration Service. | |
| The [CONFIDENTIAL] of the Innova Fluoro Images and the IVUS images will significantly increase the clinical value of the IVUS [CONFIDENTIAL] to the End User, thus could represent a higher value proposition for VC’s IVUS Catheters offering, driving usage and/or catheters pricing. | ||
| [CONFIDENTIAL] VC agrees to buy [CONFIDENTIAL] from GEHC as follows: |
| o | From the date of Products installation until the [CONFIDENTIAL] month anniversary of such Installation, VC shall purchase from GEHC [CONFIDENTIAL] for each [CONFIDENTIAL] s5i/GE or s5i units sold by GEHC (directly through GEHC or indirectly through GEHC agents, distributors and representatives) | |||
| o | Passing the [CONFIDENTIAL] month anniversary of a s5i/GE or s5i installation, GEHC agrees to provide [CONFIDENTIAL] to VC for [CONFIDENTIAL]. | |||
| o | For the purpose of establishing the number of [CONFIDENTIAL] to be purchased by VC, VC shall be responsible for consolidating the number of [CONFIDENTIAL] by Country, Region and provide a quarterly reporting to GEHC. VC may also use the number of [CONFIDENTIAL]. |
17/39
| o | Quarterly, VC shall pay GEHC for the corresponding number of [CONFIDENTIAL]. | |||
| o | In countries where ▇▇ ▇▇▇▇▇▇ it impractical to manually consolidate the [CONFIDENTIAL]. VC shall establish a report based on [CONFIDENTIAL] . | |||
| o | For the purpose of consolidating the [CONFIDENTIAL]. | |||
| o | GEHC shall have the right to audit [CONFIDENTIAL]. For countries where such [CONFIDENTIAL] does not exist, GEHC shall have the audit rights over VC records relating to the calculation of the [CONFIDENTIAL]. | |||
| o | Parties acknowledge that all of the s5i and s5i/GE systems contemplated in this agreement are to be completed as [CONFIDENTIAL]. Units placed on “[CONFIDENTIAL]” or “[CONFIDENTIAL]” shall not be allowed as part of this formal agreement. In situations where a customer insists on a [CONFIDENTIAL] agreement for the s5i/GE (not an s5i) configuration in a GE cath lab, VC and GEHC may mutually agree to offer a [CONFIDENTIAL] agreement (under a VC catalogue number, not a GEHC catalogue number). In this case, VC will have all responsibility for the sales process and system installation, warranty and start-up (which may be subcontracted to GE) and a fixed, on-time, unlimited [CONFIDENTIAL] would be paid to GEHC as follows: $[CONFIDENTIAL]. at time of installation and $ [CONFIDENTIAL] 12 months following installation (due on s5i/GE only). Note that since the s5i/GE would be delivered under a VC catalogue number, no [CONFIDENTIAL] would be due to GEHC on future [CONFIDENTIAL]. |
| 7.4 | Software/Firmware. The Price of all Pilots and Products include a perpetual, paid- up, worldwide, irrevocable license for GEHC, GEHC Group, its End-Users, its customers, Representatives to use in the operation, maintenance and repair of the Products any related software/firmware which is furnished to GEHC, GEHC Group, its End-Users, Representatives or other users of GEHC products containing the Products. | |
| 7.5 | Cost Reductions. During the Term of the Agreement, GEHC and VC shall undertake a program to achieve reductions in the cost of Products by utilizing cost-effective design, lower cost components, new technology, alternative sources, specification or features trade-off, productivity improvements and automation of the manufacturing process. To assist each other in this joint program, GEHC and VC shall determine the feasibility and potential savings from alternative actions, and shall share (as necessary) the required cost data, expertise in selecting materials, components, alternative sources and manufacturing processes for analysis. VC and GEHC shall use their commercially reasonable efforts in this joint cost reduction |
18/39
| program to further reduce the Prices by at least [CONFIDENTIAL] a year without altering VC margin. Any saving resulting from any cost reduction program specifically enabled by virtue of GEHC’s purchasing power, buying agreements, partnerships with other common vendor, etc.. will result into a [CONFIDENTIAL] allocation of the savings to VC, and a [CONFIDENTIAL] allocation of the savings to GEHC in the form of a reduction of the Prices given in Section 7.1 . . Furthermore, any saving resulting from any cost reduction program identified and pursued by VC alone or by collaboration between VC and GEHC that also results in material changes affecting fit / function / form, Service, Reliability, Functionality of the Product which, in turn, force GEHC to dedicate resources to re-qualify the Product and/or its spare parts and/or re-train field service personnel will result into a [CONFIDENTIAL] allocation of the savings to VC, and a [CONFIDENTIAL] allocation of the savings to GEHC in the form of a reduction of the Prices given in Section 7.1. Such [CONFIDENTIAL] allocation to GEHC is intended as consideration / reimbursement for required dedication of resources to adapt to product changes. For the avoidance of doubt, any cost reduction which is pursued by VC alone or together with GEHC that does not affect fit / function / form, Service, Reliability, Functionality and does not require re-qualification of the Product or changes in spare parts or field service training by GEHC would result in [CONFIDENTIAL] of the cost savings being allocated to VC. |
| 8.1. | Contents. A purchase order released by GEHC for Products or spare parts (“Purchase Orders”) may consist of a hard copy in the form set forth in Attachment F, an electronic message or other written communication from GEHC to VC, which complies with the requirements of this Agreement. Purchase Orders released by GEHC are subject to the terms and conditions of this Agreement and are considered an integrate part thereof, and shall identify the delivery date or dates and the quantities to be released for Delivery within the lead times specified in Article 6 or in Attachment D. REGARDLESS OF FORM, EVERY PURCHASE ORDER IS DEEMED TO INCLUDE GEHC’ STANDARD TERMS AND CONDITIONS OF PURCHASE SET FORTH IN ATTACHMENT F. | |
| Notwithstanding anything else to the contrary in this Agreement, if a Purchase Order released by GEHC already contains or refers to Standard Terms and Conditions of Purchase, such Standard Terms and Conditions of Purchase shall prevail over Attachment F. | ||
| 8.2. | Acceptance. Within 2 business days from receipt of a Purchase Order, VC shall acknowledge receipt of such Order by ensuring it has the necessary stock of component parts for its own production, and confirming by any mean defined by GEHC, the delivery date and quantity for the ordered items. VC may not reject any Purchase Order of GEHC unless, in accepting a Purchase Order, VC would violate any law or regulatory body | |
| 8.3. | Changes. Subject to Article 6, GEHC may change the quantities, configurations, and delivery dates on individual Purchase Orders for Products without penalty as long as GEHC notifies VC of the changes at least two weeks prior to Delivery. |
19/39
| 9.1. | Customer Copies. Unless agreed otherwise in writing by GEHC, each Product delivered by VC shall include a Pre-installation ▇▇▇▇▇▇ in English, a set of Operator Manuals in English, French, German, Spanish and any additional language as defined in Article 4, a set of Service Manuals in English, containing all applicable drawings, schematics, software license(s), software documentation, spare part lists, theory of operation, service troubleshooting diagnostics, and instructions necessary for the installation, operation, maintenance and repair of the Product. The documentation shall be in a format acceptable to GEHC. | |
| 9.2. | GEHC Master Copy. VC shall provide to GEHC at no additional charge a complete set of reproducible master copies of all documentation listed above, which GEHC may reproduce without charge. If any change in the Product requires a change in the documentation, VC shall promptly notify GEHC of the change and provide a revised reproducible master copy without charge. |
| 10.1 | VC shall provide any necessary personnel and equipment to train GEHC’ employees, Representatives with respect to the installation, operation, maintenance and repair of the Products. Such training shall be at VC’s facility and be provided at no cost to GEHC, for a maximum of 10 persons at a time and for maximum of 6 sessions a year. | |
| 10.2 | VC shall not pay for travel and living expenses incurred by GEHC employees, and those of its Representatives. If VC provides training at GEHC’ designated location (other than VC’s facility), GEHC shall reimburse VC for the reasonable travel and living expenses incurred by VC’s personnel to conduct such training at such location; provided, however, that such reimbursement shall be limited to the amount estimated by VC, and approved by GEHC in advance. |
| 11.1. | Testing. VC shall test all repaired Products and spare parts using the highest quality test plan or procedure used by VC to test products similar to the Products. |
20/39
| 11.2. | Duration. For 10 (ten) years after the last manufacturing date of the Product, VC shall maintain the capability of repairing Products (including the know-how to repair spare parts and qualified employees) and/or of furnishing replacement products, and of furnishing all spare parts, service tools, documentation and instruments necessary to effectively service the Product(s). As rapid technology evolution may make maintaining 10 years of spare parts impossible, VC agrees to supply either complete sub-assemblies or new units that are functionally equivalent, where appropriate. | |
| 11.3. | If a spare part becomes obsolete impeding VC from supplying such spare part to GEHC for the remaining of the 10 years period as defined in Section 11.2, VC is committed, in the order of priority to (i) inform GEHC by written notice with acknowledgement of receipt the obsolescence of such Spare part which shall not be earlier than 6 months from such notice, (ii) propose to GEHC a replacing product fully compatible (regarding fit, form and function) with the Spare part concerned (iii) propose to GEHC the name of another supplier having the ability and the desire to manufacture the Spare part concerned if possible, or (iv) propose to GEHC a last buy which conditions (price, delivery date, etc.) will have to be agreed by both Parties, and (v) to promptly transfer to GEHC all instruments, tools and documentation necessary to service such spare part(s). | |
| 11.4. | Emergency: In case of emergency (e.g.. if GEHC does not have any more spare parts available), VC shall make it commercially reasonable efforts to deliver any Spare part for the Product within 24 hours of GEHC’ request and send it to the location designated by GEHC. | |
| 11.5. | Spare parts Pricing. Spare parts list, corresponding pricing, lead-time and reparability should be agreed upon between GEHC and VC prior to entering in the Full Production Phase. Such list and pricing shall be listed in Attachment D. Prices for spare parts should be defined in such a way that the total Prices of all spare parts necessary to build one Product should not exceed USD [CONFIDENTIAL]. | |
| 11.6. | VC shall repair any spare part returned by GEHC within one (1) week from reception date. | |
| 11.7. | Field related complaints. Throughout the duration of this Agreement and for ten years following the last manufacturing date of the Product, VC shall perform the second level of support to GEHC and handle field related complaints (recurrent technical issues, technical related Customer complaints, product safety issues) related to the Products. This activity will consist into analyzing, developing and implementing technical fixes and closing field complaints related to the Product as communicated by GEHC to VC: |
| Ÿ | PQRs: Significant Quality or technical issues: |
| o VC shall complete the assessment within 5 Days from notification | |||
| o VC shall do its commercially reasonable effort to implement a fix within 30 Days from notification |
| Ÿ | CSOs: Customer Satisfaction Issue |
| o VC shall complete the assessment within 5 Days from notification |
21/39
| o VC shall do its commercially reasonable effort to implement a fix within 30 Days from notification |
| Ÿ | PSRs: Safety issues |
| o shall complete the assessment within 2 Days from notification | |||
| o VC shall do its commercially reasonable effort to implement a fix within 30 Days from notification |
22/39
| 13.1 | Content of Invoice. VC’s invoices shall contain the Purchase Order release number, item number on such release, gehc part number, invoice quantity, unit of measure, unit price, total invoice amount, (excluding VAT), VAT amount, total invoice amount (including VAT), VAT registration number, name of VC, phone number, address to which remittance should be sent, and such other information as may be required by GEHC. | ||
| 13.2 | Payment by GEHC. | ||
| Subject to Article 5 and 6, during the Production Phase, GEHC shall settle the invoice within [CONFIDENTIAL] days from invoice date after receiving both the orders items and an invoice prepared in accordance with the terms of this Agreement. | |||
| 13.3 | Payment by VC of GEHC’s Image Co-Registration Services. | ||
| Subject to Section 7.3, VC shall settle the payment of Image Co-Registration Services due to GEHC within [CONFIDENTIAL] days following close of Quarter. Payments shall be made to: |
GE Healthcare Technology
Interventional Cardiology and Surgery Business
▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇▇ ▇▇▇▇▇
▇▇▇▇▇▇▇▇▇, ▇▇▇
| 14.1. | Terms. VC warrants that the Products to be delivered under the Agreement will be free from defects in material, manufacturing workmanship, and title. Product warranties and all remedies for warranty failures are granted for a period of eighteen (18) months from Product delivery date or twelve (12) months from Product installation date, Which ever occurs the first. Any goods or parts furnished to GEHC during the warranty period of a Product to correct a warranty failure in the said Product shall be warranted for an additional eighteen (18) month period from parts delivery or three (3) months from part installation date, which ever occurs the first. | |
| 14.2 | Return of Non-conforming or Defective part of a Product. | |
| GEHC may return any non-conforming or defective part of a Product under warranty to VC with VC prior authorization. In such case, any such part of a Product shall be returned to VC’s facility or authorized service center, with all transportation charges paid by VC and the risk of loss passing to VC when the part of the Product is delivered to the carrier of VC’ choice. If GEHC elect to return any non-conforming or defective part of a Product under warranty to VC without VC prior authorization, transportation charges shall be paid by GEHC and the risk of loss passing to VC when the part of the Product is delivered to VC. | ||
| 14.3. | Repairs / Replacement / Credit. If any Product or part thereof is found to be defective or non-conforming during the warranty period (Defective Part), VC shall within five (5) working days from the date of reception of the Defective Part in its premises unless a longer period is mutually agreed upon, repair or replace the Defective Part. The list of repairable Parts shall be agreed upon between the Parties. |
23/39
| 14.4 | Trouble Shooting Report. For any claim under warranty and for all repairable parts (as defined in Attachment D), GEHC shall attach a troubleshooting report detailing the findings, Product history (Serial number and installation date of the Product, Serial Number of the Defective Part) and all information required to define if the warranty was still applicable when the failure occurred. As part of the program, a troubleshooting report template will be developed and agreed upon by GEHC and VC. It shall include the proper warranty conditions allowing GEHC Field Engineer to only return spare parts under warranty. A blank template of this troubleshooting report shall be included with all returnable (all parts with a unit price higher than $500) or repairable spare parts as defined in Attachment D supplied by VC to GEHC. Spare Parts shall be labeled with the appropriate level of information (Manufacturing date or Serial Number) to allow GEHC or VC to establish if a defective part is still under warranty. | |
| 14.5 | Emergency: In case of emergency and upon GEHC notification, VC shall be able to ship at GEHC designated location, a replacement for a non-conforming or Defective Part of a Product under warranty. If the corresponding non-conforming or Defective Part has not been returned by GEHC to VC within thirty (30) days from GEHC’ notification, VC shall be allowed to invoice GEHC for the Price of such part. | |
| 14.6 | All repaired of replaced spare parts to be sent to GEHC, should be processed in accordance with Attachment H. |
| 15.1. | Product Quality. VC is committed to quality in the performance of this Agreement, and acknowledges that the delivery of quality Products is of the essence to this Agreement. All Products and spare parts shall be manufactured in accordance with the following principles: | |
| The quality goal for all Products at the time of installation at an End-User location is zero “Failure On Installation” Product (“FOl”). A FOI Product is a Product that fails (does not meet the Purchase Specifications) within one week of turn over of the Product to an End-User. | ||
| The quality goal for all spare parts at the time of a service intervention at an End-User location is zero “Failure On Arrival” Spare part (“FOA”). A FOA Spare part is a Spare part that fails within one week from service intervention. |
24/39
| 15.2 | Product Reliability. The Mean Time Between Failure (Herein referred to as “MTBF”) for the Products shall not be less than [CONFIDENTIAL] for the System, a failure being defined as a Malfunction of the software or hardware which totally prevents the use of the system for its fundamental clinical usage and when such malfunction cannot be recovered unless making the parts replacement / software upgrade by the authorized service engineers. Failures caused by normal wear and tear and user-induced damage will not be subject to this measurement. If the Reliability of the Products is lower than the above minimum targets, VC shall promptly submit to GEHC a written corrective action plan, which at a minimum contains an analysis of the root cause(s) and specific actions taken or planned to correct the reliability weakness, including but not limited to the development of an FMI as defined in Section 4.1. | |
| 15.3 | Late Deliveries. VC acknowledges that timely delivery of conforming Products is of the essence of this Agreement. VC shall notify GEHC immediately if VC ever has reason to believe that any Product will not be delivered as ordered, or a shipment will not be made as scheduled. In connection with this notification: | |
| The delivery goal for all Products is 100% on-time delivery. This rate shall be calculated periodically by GEHC as the number of on-time deliveries Ex-Works VC Facility or within five days prior to the scheduled delivery date during a rolling three month period divided by the total number of deliveries during the same period. If on-time delivery falls below 95%, VC shall consult with GEHC and promptly develop a corrective action plan satisfactory to GEHC in its reasonable discretion. | ||
| Notwithstanding anything to the contrary herein, If VC on-time delivery falls below 80%, GEHC may, without incurring any liability or cancellation fee, partially or totally cancel its orders for the Products late for Delivery by more than 5 days from the originally scheduled delivery date, unless the delay is due to GEHC’ breach of this Agreement or Force Majeure. |
25/39
| 16.1. | General. VC shall comply with the GE’s Integrity Policies, a copy of which is attached as Attachment E, and all applicable laws and regulations in the manufacturing and delivery of the Products or in otherwise performing any of its obligations under or in connection with this Agreement or any other agreements. Such laws may include, but not limited to, United States, Danish, French and foreign medical device laws, regulations and directives, labor laws, environmental laws, Customs Trade Partnership Against Terrorism (CTPAT) regulation and product safety laws. VC further represents and warrants that: (i) VC and its suppliers will not use child, forced of prison labor in connection with the manufacture and supply of Products; (ii) VC and Its employees will not offer gifts, bribes, kickbacks, free travel or other cash or non-cash incentives to GEHC’ employees. VC will provide GEHC all information necessary to enable GEHC to comply with the laws and regulations applicable to GEHC’ use of Products. | |
| 16.2. | VC will at its own expense, apply for and obtain all regulatory approvals from the FDA and all other governmental and regulatory approvals, to the extent required to market, sell and distribute the Products, and comply with the most current governmental and other regulatory standards with respect to the Products as may be supplemented or revised from time to time. VC will be responsible for coordinating the efforts and taking the lead with respect to such approvals. To the extent permitted by law, all regulatory approvals obtained by VC and GEHC related to the Products shall be made in VC’s name and, jointly in GEHC’ and VC’ name, where necessary for GEHC to be able to promote, distribute and support the Products | |
| 16.3. | Product Certification. VC shall manufacture the Products in strict compliance with all applicable listings such as CE, UL, IEC, CSA and FDA/Quality Safety Regulatory (for medical devices), SFDA, CCC or equivalent listing and maintain the same at its expense. Unless otherwise agreed to in writing, if a Party proposes a change in purchase specifications pursuant to Article 17, such Party shall be responsible for any additional product certification costs that may be necessary. Upon GEHC’ request, VC shall provide GEHC with a copy of all regulatory certification reports. VC shall also comply, at its own costs, with international quality standards such as ISO 9001:2000 and ISO 13485. VC shall maintain all above mentioned files and listing according to specifications in a continuous way and communicate same, upon request to GEHC, for the period required by applicable laws so as to enable GEHC never to be in breach of such laws pursuant to which the applicable authorities may require during the lifetime of the Products evidence of such Products certification and listings. | |
| 16.4. | Medical Device Reporting. Pursuant to the FDA’s and other European and foreign applicable Medical Device Reporting regulations, if either Party is required to report to the FDA or European notified bodies information that reasonably suggests that a Product may have caused or contributed to the death or serious injury or has malfunctioned and that the device would be likely to cause or contribute to a death or serious injury if the malfunction were to recur then each of VC and GEHC agree to supply to the other any such information promptly after becoming aware of it so that each of VC and GEHC can comply with governmental reporting requirements. Each Party will use commercially reasonable efforts to comply with all applicable |
26/39
| 16.5. | Customs Compliance. VC agrees to provide GEHC with all necessary information and assistance to complete the Product’s Data Records («PDR») and all other customs documentation required by law. This information shall include, without being limited to, the Product, spare parts or Option descriptions and characteristics, part number, dimensions, weight, dangerous goods information, country of origin, name of Original Equipment Manufacturer («OEM») |
| 17.1. | GEHC-Proposed Changes. GEHC may propose changes to the Purchase Specifications or to the Product or part thereof by submitting the proposed changes (identifying those changes which it deems mandatory to make the Product suitable for use) to the Agreement Manager of VC, utilizing the Product Change Notice (PCN). VC shall respond in writing to the agreement manager of GEHC within ten (10) days after receipt of such changes with the following information, as applicable: (i) lead time required to implement proposed changes; (ii) impact of proposed changes on pricing of Product, including parts and tools; (iii) impact of proposed changes on scrap material and work in process; and (iv) non-recurring engineering charges to implement proposed changes. Within no more than ten (10) days after GEHC receives VC’s response to GEHC’ proposed changes, the Parties shall begin negotiations with respect to the above changes and any related changes to the price and delivery schedules. If the Parties fail to negotiate appropriate changes to the Agreement, the terms in effect prior to the commencement of the negotiations shall remain in full force and effect. | |
| 17.2 | VC-Proposed Changes. VC may not make any changes to the Product affecting form, fit, function, reliability, serviceability performance, functional interchangeability, options or spare parts interchangeability or interface capability without obtaining GEHC’ prior written approval by submitting a change notice, at least ninety (90) days before the change Is implemented. If VC makes any such change to a Product without GEHC’ written approval, VC shall be responsible for the costs incurred by GEHC in order to fix such issue. When GEHC realize that it will have to incur some major costs or expenses due to some VC unproved changes, GEHC shall not engage major costs and expenses without immediately notifying VC, and the Parties shall define the best course of action to repair or replace the affected products or part thereof at GEHC’ facility or GEHC’ End-User site regardless of whether the Product is in or out of warranty. VC shall then be responsible for the corresponding direct costs, expenses or damages, labor and material costs to be further incurred by GEHC (excluding indirect damages or lost of revenues) in order to repair the affected Products. |
27/39
| 18.1. | Access. GEHC, in its sole discretion, may permit VC to have on-line access to designated computer systems of GEHC in order to facilitate VC’s ability to perform its obligations under this Agreement. If such access is granted, VC shall give to GEHC the names of VC’s employees who have a legitimate business need for such access to GEHC’ computer systems, and GEHC shall provide a separate user identification code for each person. VC, at its own expense, shall provide and maintain any hardware, telecommunications services and software not furnished by GEHC, which are needed to communicate reliably with GEHC’ computer systems. GEHC, in its sole discretion, may terminate VC’s access to GEHC, computer network at any time. | |
| 18.2. | Use Restrictions. VC shall ensure that: (i) computer access is limited to those employees with a legitimate business need whose names have been furnished to GEHC; and (ii) such employees with access agree to keep any information so obtained strictly confidential, to use such information only to perform VC’s contract obligations to GEHC and to cease accessing GEHC’ computer systems when no longer required to perform work under this Agreement. VC shall promptly notify GEHC if it becomes aware of any unauthorized access to GEHC’ computer systems or unauthorized use of the information on the systems. | |
| 18.3. | Legal Effect. Any document properly transmitted by computer access shall be considered a writing delivered in connection with this Agreement. Electronic documents shall be considered signed by a Party if they contain an agreed upon electronic identification symbol or code. Electronic documents shall be deemed received by a party when accessible by the recipient on the computer system. Electronic documents that can possibly lead to one Party’s material breach of this Agreement, shall be acknowledged by the receiving Party. |
28/39
| 20.1 | Ownership of Pre-Existing VC Intellectual Property. Subject to the license and ownership rights set forth in this Agreement, sole and exclusive right title and interest to current VC products, all Patents and all other intellectual property rights associated with the current VC products, including any copyrights or trade secrets, shall remain with VC (“Pre-existing VC Intellectual Property”). | ||
| 20.2 | Ownership of GEHC Intellectual Property. Sole and exclusive right, title and interest to all GEHC products and any intellectual property contained therein or related thereto, including any patents, copyrights or trade secrets, shall remain with GEHC (“Pre-existing GEHC Intellectual Property”). | ||
| 20.3 | Ownership of Inventions and Improvements | ||
29/39
| (i) | VC Inventions. VC shall have and retain sole and exclusive, right, title and interest to all inventions, improvements, discoveries and know-how which, during the term of this Agreement, are solely made by, or solely at the expense of, VC during the term of this Agreement are solely made by, or solely at the expense of VC, its employees, sublicensees, contractors or agents acting under authority from VC (“VC Inventions”, together with all intellectual property rights associated therewith and together with the Pre-existing VC Intellectual Property, the “VC Intellectual Property”). | ||
| (ii) | GEHC Inventions. GEHC shall have and retain sole and exclusive right, title and interest to all products, inventions, improvements, adaptations, enhancements, interfaces, documentation, discoveries and know-how which, during the term of this Agreement, are solely made by, or solely at the expense of, GEHC, its employees, sublicensees, contractors or agents acting under authority from GEHC (“GEHC Inventions”, together with all intellectual property rights associated therewith and together with the Pre-existing GEHC Intellectual Property, the “GEHC Intellectual Property”). | ||
| (iii) | Joint Inventions. In the event that VC and GEHC desire to engage in the joint development of a new product / feature (“Joint Inventions”), VC and GEHC shall negotiate in good faith to agree upon the terms under which the parties will jointly develop such Joint Invention. Including, without limitation the allocation of development expenses and responsibilities, maintenance and support responsibilities, joint ownership of intellectual property rights, with both parties having unrestricted royalty-free rights to use the technology. There shall be reciprocal indemnity for intellectual property contributed by the other party plus maximum cap on intellectual property liability commensurate with each party’s potential upside benefit. The parties shall use their reasonable efforts to agree upon reasonable terms governing the treatment of Joint Inventions within thirty (30) days of electing to create Joint Inventions. | ||
| (iv) | Subject to Section 20.3.(i) and 20.3 (ii), and unless agreed otherwise during the Term of this Agreement, VC and GEHC shall have joint rights over jointly developed improvements and enhancements to the Product. |
| 21.1 | All Necessary License Rights. Subject to the provisions of this Agreement, including Section 21.2 herein, VC hereby grants to GEHC all license rights to any of the VC Intellectual Property necessary to allow GEHC to perform its obligations and exercise its rights under this Agreement, including but not limited to those set forth in Article 2 above. The Term of each license granted under this Section 21.1 shall be concurrent with the Term, but shall survive indefinitely and irrevocably with respect to Products marketed, distributed and sold prior to termination of this Agreement or as provided under Section 22.4. |
| (i) | Subject to Section 21.2 (ii) below, VC hereby grants to GEHC a non-exclusive, fully-paid royalty free, perpetual license (with right to sublicense, the “License”) to VC Intellectual property, to use, execute, reproduce, display, perform, distribute, modify, create derivative works of, make, have made, market, offer for sale, sell, import and sub-license the Products land parts thereof) incorporating, or covered; by, VC Intellectual Property. | ||
| (ii) | The License shall only become effective in the event that (i) if VC ceases operations or Files for Bankruptcy without appointing a successor reasonably acceptable to GEHC, or (ii) if VC materially and repeatedly breaches its maintenance and support obligations under Article 11 thus preventing GEHC to service existing installations of the Products. In addition, the parties agree that if this Agreement is terminated or rejected by a party in bankruptcy, then all rights and licenses granted under or pursuant to this Agreement shall be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code and any similar Laws in any other country in the Territory, licenses of rights to “intellectual property” as defined under U.S. Bankruptcy Code. The Parties agree that all intellectual property rights licensed hereunder, including, without limitation, any technology, software, patents or patent applications in any country of a Party covered by the license grants under this Agreement, are part of the “intellectual property” as defined under the Bankruptcy Code subject to the protections afforded the non-terminating Party under Section 365(n) of the Bankruptcy Code, and any similar law or regulation in any other country. | ||
| (iii) | Technical Information. Upon the occurrence of any of the events in Section 21.2 (ii) above, the License shall automatically become effective without the requirement of any action by either party, and VC shall promptly make available to GEHC, at no cost to GEHC, access to all source code and spare parts suppliers necessary to service all Product(s) or spare parts delivered during the Term of this Agreement. GEHC shall thereafter have full right to use such information for the sole purpose of repairing or replacing spare parts and supporting the installed Product base without any claim on VC’s part for additional compensation for such use. However, GEHC shall be responsible for the cost of the repair or replacement of spare parts by third party spare parts suppliers. |
| 21.3 | No licenses are granted, by implication, estoppel or otherwise, under any intellectual property right of GEHC under this Agreement |
| 22.1 | VC hereby covenants that it will not knowingly include in any of the Products and any parts or sub-assemblies thereof or End User documentation anything that, nor will VC knowingly enter into a situation where the sale or distribution by GEHC of any of the Products or End User documentation as approved by VC, or the use of such products or documentation by GEHC’ End Users, infringes, or includes any product or parts or sub-assemblies thereof that infringes, any third party’s patent, |
| 22.2 | VC warrants that the Product shall be delivered free of any claim of third parties for infringement of any intellectual property rights patent or copyright. VC, at its own expense, shall defend, indemnify and hold harmless GEHC and its directors, officers, employees, agents, successors and assigns, and defend any action brought against same with respect to any claim, demand, debt, liability, damage, cost, loss or expense, including attorneys’ fees and expenses, based on a claim that the development, promotion, manufacture, use, sale or other disposition of, or servicing of the Product furnished under this Agreement, by VC, infringes or violates any patent, copyright, trade secret, license, or other proprietary right of any third party. VC shall control such defense and all negotiations relative to the settlement of any such claim; provided, however, that VC shall not enter into any settlement or compromise that adversely affects any rights of or imposes any obligation or liability on GEHC without the prior consent of GEHC. Upon VC’s request and at VC’s expense, GEHC will provide VC with the assistance, information, and authority reasonably necessary to perform the above defense, and VC shall reimburse GEHC for reasonable out-of-pocket and legal expenses incurred in providing such assistance. GEHC shall promptly provide VC with written notice of any claim which GEHC believes fails within the scope of this Article; provided, however, that failure by GEHC to provide such notice shall not affect VC’s obligations under this Article to the extent that VC is not materially prejudiced thereby. |
| 22.3. | At any time after VC becomes aware of any such claim but prior to any injunction or final resolution of such claim, GE may request VC use commercially reasonable efforts to procure for GEHC the right to continue to use the Product, and any portion thereof. |
| 22.4. | In the event that the Product, or any portion thereof, is held to constitute an infringement and its use is enjoined, VC shall have the obligation to, at its option and at its own expense: (i) modify the infringing Product without impairing in any respect the functionality or performance, so that it is non-infringing; (ii) procure for GEHC the right to continue to use the infringing Product; or (iii) replace the Product with an equally suitable, non-infringing replacement subject to GEHC reasonable acceptance testing. If none of the foregoing alternatives are available to VC, GEHC shall receive, in addition to its rights and remedies available to it under this Agreement and pursuant to law, a repayment of all monies paid to VC pursuant to this Agreement, plus any costs incurred by GEHC in the removal of such Product and installation of alternative products, and VC shall accept return of the Products at its expense, once GEHC has arranged for the continuation of the functions performed thereby. |
| 23.1 | Term. The term of this Agreement is from the date first written above, until December, 31 2009, (the “Initial Term”). An Extension Term may be executed if there is mutual agreement with the Parties. |
| 23.2 | Termination of Purchase Orders. GEHC may terminate any Purchase Order in whole or in part at any time upon written notice to VC pursuant to the provisions of Attachment F. GEHC’ maximum liability with respect to the terminated Purchase Order shall correspond to the material commitments pursuant to Article 6 |
| (i) | If there is a material breach of any term of this Agreement by VC and VC fails to correct such breach within ninety (90) days after receiving written notice of such breach from GEHC, GEHC may terminate this Agreement and all unfilled Purchase Orders without any liability except for the payment of any Products previously ordered by and delivered to GEHC (subject to any set-off/compensation/credit available to GEHC). | ||
| (ii) | GEHC may also terminate this Agreement immediately by giving written notice to VC if VC foils to meet its financial obligations as they become due, or Files for Bankruptcy or if VC is unable to supply the Products because of a decision to exit the business. Any such termination shall not relieve VC of its obligations and GEHC shall retain all legal and equitable remedies after such termination. A material breach by VC shall include, without limitation, the failure by VC to comply with any Attachment and with applicable product quality and delivery obligations. | ||
| (iii) | In the event that GEHC fails to purchase and install at least [CONFIDENTIAL] s5i and s5i/GE systems (taken together) for any 12-month period (rolling) from the first production shipment of the s5i/GE to GEHC (for reasons not related to pattern of repeated channel conflict issues between the Parties which materially impact GEHC’s ability to meet these criteria, where in such case the Parties shall seek resolution) both VC and GEHC shall have the option to unilaterally terminate this Agreement (with 30 days notice and cure period). In the event of such termination: |
| • | All distribution rights shall terminate (for s5i and s5i/GE) | ||
| • | Certain terms of the agreement will survive per Section 23.6 | ||
| • | VC shall fulfill GEHC’s order backlog and all End User orders resulting from quotes issued by GEHC prior to the Termination of the Agreement. | ||
| • | Accrued and future Image Co-Registration Services per Section 7.3 due to GEHC for systems installed prior to termination date shall remain as per this Agreement. |
| 23.4 | Fulfillment of Orders upon Termination. Upon termination of this Agreement by GEHC or VC, GEHC will have the right, at its sole option, to require VC to satisfy VC’s obligations under all outstanding Purchase Orders that have been accepted by VC prior to the effective date of termination, as well as fulfill GEHC End User orders |
| 23.5. | Effects of Termination. Termination or expiration of this Agreement will not relieve either Party of obligations incurred prior to the termination. |
| 23.6 | Survival of Certain Terms. The provisions of Article 1, 7.3, 11, 16, 19, 20, 21, 22, 23 (only to the extent necessary to give effect to this Section 23.6), 24, 27, and 28. will survive the termination or expiration of this Agreement for any reason along with any obligations which by nature and as per this Agreement are deemed to survive its termination and expiration. |
| 26.1 | Notices. Any notice required under this Agreement shall be sent by fax (with the
original to promptly follow by applicable national mail service or a nationally recognized
overnight courier), by a nationally recognized overnight courier, or
transmitted electronically pursuant to the terms of Article 18. Notices will be deemed given on the date
delivered to the recipient if sent by fax or overnight courier (it being agreed that the
sender shall retain proof of transmission or delivery, as the case
may be) or when accessible
electronically if sent electronically under Article 18. Notices
shall be sent to the
persons identified below (or as otherwise directed in writing by a
party): GEHC: Attention: GE Medical Systems — GM Vascular Marketing VC: Attention: Volcano Corporation — CEO |
| 27.1 | The present Agreement will be governed and interpreted by the State of New York Law, without giving effect to its conflict of laws principles. |
| 27.2 | All disputes between the parties in connection with, or arising out of, the existence, validity, construction, performance and termination of this Agreement or any terms whatsoever thereof, which the parties are unable to solve amicably between themselves, shall be settled by binding arbitration under the Rules of Conciliation and Arbitration of the International Chamber of Commerce (ICC). Any such arbitration shall be conducted in the English language, and the venue of such arbitration shall be New York City, NY, USA. |
| 28.1 | Scope. THIS AGREEMENT AND ITS ATTACHMENTS STATE THE TERMS AND CONDITIONS UNDER WHICH VC SHALL SELL TO GEHC, AND GEHC SHALL PURCHASE FROM VC ON A NON EXCLUSIVE BASIS, THOSE PRODUCTS IDENTIFIED IN ATTACHMENT A, AS WELL AS ALL COMPONENTS, SPARE PARTS, SERVICE TOOLS, MANUALS, SOFTWARE LICENSES, DATA AND RELATED INTERFACES WITH RESPECT THERETO. UNLESS OTHERWISE EXPRESSLY STATED, REFERENCES TO THIS “AGREEMENT” INCLUDE ALL ATTACHMENTS. |
| 28.2 | Parties. VC expressly acknowledges that this Agreement is not intended to govern or obligate any business, division or affiliate of GEHC other than GE Medical Systems business, and that the term “GEHC” as used herein shall refer to the GE Medical Systems business and to no other business, division or affiliate of General Electric Company. |
| 28.3 | Documents. The following attachments are an integral part of this Agreement (the “Attachments”). The provisions of each Attachment shall be incorporated by reference into and be deemed to be a part of this Agreement. If any conflict exists between the provisions of this Agreement and of the Attachments and documents mentioned below, or between the provisions of Attachments and documents mentioned below, the order of precedence shall be: |
| 28.4 | Severability: In the event any provision of this Agreement, or portion thereof, shall be deemed invalid or unenforceable by any court or governmental, agency, such provision shall be enforced to the maximum extent permissible so as to effect the intent of the parties. All remaining provisions shall continue in full force and effect. |
| 28.5 | No Waiver: The waiver or failure of either Party to exercise any right or remedy in any respect provided for herein shall not be deemed a waiver of any further right or remedy hereunder. |
| 28.6 | Audit Right: At GEHC’ request, VC will allow GEHC to inspect without charge and to copy at GEHC’ expense any documents you have relating to the performance of VC’s obligations under this Agreement. |
| 28.7 | Except as permitted pursuant to Article 24, VC and GEHC shall not issue any press release or announcement, use any of each others’ products or its name in promotional activity, or otherwise publicly announce or comment on this Agreement without GEHC’ or VC’ prior written consent.” |
| 28.8 | This Agreement, may not be assigned by VC or GEHC without the express written consent of GEHC on the one hand and VC on the other. |
| 28.9 | This Agreement may be modified only by a writing signed by the Agreement Managers for both Parties. |
GEHC
|
VC | |||||||||
By:
|
/s/ ▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
|
By: | /s/ ▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇
|
|||||||
| Name: ▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇ | Name: ▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ | |||||||||
| Title: Modality Sourcing Leader ICS | Title: President & CEO | |||||||||
GEHC
|
VC | |||||||||
By:
|
/s/ ▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ | By: | ||||||||
| Name: ▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ | Name: | |||||||||
| Title: GM Sourcing Europe | Title: | |||||||||
Attachment B (Purchase Specifications — Factory Acceptance Test)
Attachment D (Spare parts Pricing Schedules and Lead-times)
Attachment E (GE Integrity Policies)
Attachment F (Standard Terms and Conditions)
Attachment G (Product Change Notice Form)
Attachment H (Repair Information and Service Packaging)
Attachment I (Purchased Material Quality Requirements)
Attachment J (Initial Orders for Pilots)
Attachment L (Prices for the Products)
Attachment M (Countries of the Territory that Currently Maintain Exclusive Distribution agreements with VC)
Attachment N (EU language policy)
Product description, configuration, datasheet and/or brochure
| s5i, Marketing Design | ||||
|
Input | |||
| Control Number: 806105001/002 |
| Marketing Product Manager: ▇▇▇▇▇ ▇▇▇▇▇ Pool | Date: September 30, 2005 |
| s5i, Marketing Design | ||||
|
Input | |||
| Control Number: 806105001/002 |
| 1. | Design and develop an IVUS system to be permanently mounted in the Cath Lab for use at the patient table | ||
| 2. | Provide a system that can drive remote external LCD, Plasma and CRT displays |
||
| 3. | Provide for remote control panel operation in the Cath Lab and/or the Control Room | ||
| 4. | Provide for attaching up to two remote image displays from the s5ii system | ||
| 5. | Utilize same hardware as s5i to create s5p and s5g systems |
| Customer’s desire: | |||
| 5.1 | Traditional IVUS capabilities | ||
| 5.2 | Physically integrated into the cardiac catheterization lab | ||
| s5i, Marketing Design | ||||
|
Input | |||
| Control Number: 806105001/002 |
| 5.3 | A simple to operate product | ||
| 5.4 | Application quickly available | ||
| 5.5 | Little or no training to operate the product | ||
| 5.6 | The ability to document and store the data from the procedure | ||
| 5.7 | A clear/crisp image | ||
| 5.8 | The ability to bookmark interesting areas |
| 5.1.1 | System will support combination tomographic and sagittal views. |
| o | Tomographic IVUS image. | ||
| o | Sagittal view either vertically or horizontally simultaneous with tomographic display. | ||
| o | All measurements need to be displayed on imaging views. | ||
| o | Data capture and display: record video loops and still images | ||
| o | Ability to replay or review captured images Data capture and display |
||
| o | Record video loops and still images |
| 5.1.2 | Display information: |
| o | Display in English | ||
| o | Additional languages include: French, Italian, German, Spanish languages - deferred to version 2 | ||
| o | Volcano logo | ||
| o | Patient demographic information | ||
| o | Current date, time, software version. | ||
| o | Patient co-morbidity data available in the patient screen. |
| 5.1.3 | Measurements: distances, areas, longitudinal distances, and borders. |
| 5.1.4 | Support all current Volcano cardiovascular and peripheral IVUS catheters and will have the ability and flexibility to support new catheter designs. |
| 5.1.5 | Standard local XGA video output will be provided, also 2 remote video outputs. | ||
| 5.1.6 | Output for network (DICOM), Ethernet RJ-45 connector | ||
| 5.1.7 | Will use standard Patient Interface Module, located within 30 feet of the CPU | ||
| 5.1.8 | Connector on CPU chassis to allow use of the control panel, trackball or keyboard, 2 max. | ||
| 5.1.9 | System will support communication with a new design remote archiving station for color image printing and DVD recording of patient data, 1 ▇▇▇ |
| s5i, Marketing Design | ||||
|
Input | |||
| Control Number: 806105001/002 |
| 5..2.1 | System will have all computer components and PCBs integrated into a small, reduced weight, housing, suitable for mounting on, or near the patient table in the Cath Lab and within 30 feet of the patient in the Control Room or Equipment Room. | ||
| 5..2.2 | A control panel, derived from the s5 will be mounted from the DIN rails on the patient table in the Cath Lab and/or the Control Room and connect to the CPU by a single cable. It should also be provided with mounting hardware to mount it from a roll around equipment cart or from a wall. It should have suitable rubber feet so that it can be placed on a table top without marring the finish. | ||
| 5..2.3 | Approx. CPU dimensions: 16” x 16” x 6” (H x W x D). | ||
| 5..2.4 | Approx. CPU Weight: < 30 lbs. | ||
| 5..2.5 | Approx. Control Panel Dimensions: 10” x 16” x 4” (H x W x D.) | ||
| 5..2.6 | Approx. Control Panel Weight: <10 Ibs. | ||
| 5..2.7 | The CPU must be capable of being attached to patient table. The mounting system must be designed for easy installation by a field service tech. | ||
| 5..2.8 | The Control Panel enclosure must be attachable to a boom/arm/mount that can be mounted on or to the control area of the patient table. It should also be capable of being mounted to a roll around IV pole cart. It should also be possible to place it on a flat table for desktop use. |
| 5..2.9 | Volcano Custom OEM Flat Panel Monitor: nominally 19” diagonal display area. | ||
| 5..2.10 | Monitor screen visible in reduced light cath lab environment with minimal distortion when viewed from side or off angle (up to 30 degrees off angle). | ||
| 5..2.11 | Contrast & brightness and image vertical size adjustment capability required. |
||
| 5..1.12 | The display will be mounted on an articulated arm that allows the display position to be changed from stowed under the patient table, to easily visible at the patient table. | ||
| 5..2.13 | The display should be provided with a table mount for remote display locations. |
| 5..2.12 | The Control Panel will connect to the CPU and allow operation of the s5i GUI and patient data entry using the pull out keyboard. The Control Panel can be mounted on or near the patient table, on a freestanding mobile IV pole carrier, or from an articulated wall |
| s5i, Marketing Design | ||||
|
Input | |||
| Control Number: 806105001/002 |
| mounted arm, depending on customer preferences. | |||
| 5.2.13 | A remote archiving station will be provided to permit file transfer from the Cath Lab integrated CPU and burning patient files to a DVD from a direct Ethernet connection to the CPU. It will be located in the control room. |
| 5..3.1 | Will offer an intuitive, easy to use interface with minimal menus and minimal levels of operation. | ||
| 5..3.2 | Primary operator control will be through on screen curser and screen controls on the LCD/CPU display. | ||
| 5..3.3 | Additional controls, for measurement or dialog boxes will include a trackball and a keyboard. | ||
| 5..3.4 | An archiving station will consolidate the tasks of saving data, printing data &/or networking data. |
| 5..4.1 | Application is always on. | ||
| 5..4.2 | PC Boot up time less than 120 seconds | ||
| 5..4.3 | After PC boot-up the system is ready to go imaging in less than 60 seconds or less. The software and hardware will be properly initialized and calibrated during this time. Any errors in this process will be reported to the user, such as equipment setup, calibration and catheter initialization |
| s5i, Marketing Design | ||||
|
Input | |||
| Control Number: 806105001/002 |
| 5.5.1 | Console button design will follow the typical clinician workflow – designed to be intuitive for the casual user | |
| 5.5.2 | On screen step by step (“wizard”) available |
| 5.6.1 | Primary Storage Medium: Internal CPU hard drive(s). | ||
| 5.6.2 | Secondary Storage: Media will be DVD-R disks for archival purposes. | ||
| 5.6.3 | Tertiary Storage: Hospital DICOM server via the PACS network. | ||
| 5.6.4 | Ability to review of data on systems from both primary and secondary sources. | ||
| 5.6.5 | Ability to view Volcano images on a computer, such as in their office or home | ||
| 5.6.6 | Minimum three, 90 second video loops stored @ 30fps | ||
| 5.6.7 | Will not support legacy Volcano CD-R IVUS disks |
| 5.6.9 | A remotely located archiving station will be provided that connects to the CPU by means of a direct Ethernet cable connection. | ||
| 5.6.10 | Data can be burned to a DVD-R. | ||
| 5.6.11 | The archiving station will provide a USB connector to attach a USB printer for color image hard copy. |
|
s5i, Marketing Design | |||
| Input | ||||
| Control Number: 806105001/002 |
| 5.7.1 | Image Quality: Meridian system will have grayscale IVUS imaging quality equivalent to or better than current IVG Imaging System | ||
| 5.7.2 | Ability to adjust the live image. |
| 5.8.1 | Mechanical button on the console labeled bookmark | ||
| 5.8.2 | Mechanical button sends a software command to place a ▇▇▇▇ on the ILD. | ||
| 5.8.3 | The ▇▇▇▇ will be archived and available on the archived case. |
| § Hardware operators/“Button-pushers” |
| o | RN’s | ||
| o | CV technicians | ||
| o | Volcano field personnel |
| § Decision makers/Data Interpreters |
| o | Physicians |
|
s5i-GE, Marketing Design | |||
| Input | ||||
| Control Number: 806105-002/001 | ||||
Marketing Product Manager:
|
▇▇▇▇▇ ▇▇▇▇▇ Pool | Date: December 9, 2005 | ||
1.
PROJECT NAME/NUMBER
|
S5I-GE |
Page 1 of 8
|
s5i-GE, Marketing Design | |||
| Input | ||||
| Control Number: 806105-002/001 |
| 1. | Design and develop an IVUS system to be built into a new GE Cath Lab | ||
| 2. | Provide a system that can drive remote external LCD, Plasma and CRT displays | ||
| 3. | Provide for remote control panel operation in the Cath Lab and/or the control room | ||
| 4. | Provide the capability to create a report reflecting the serial numbers of the catheters that are used with this system. |
| Customer’s desire: | |||
| 5.1 | Traditional IVUS capabilities | ||
| 5.2 | Physically integrated into a GE cardiac catheterization lab | ||
| 5.3 | A simple to operate product | ||
Page 2 of 8
|
s5i-GE, Marketing Design | |||
| Input | ||||
| Control Number:806105-002/001 |
| 5.4 | Application quickly available | ||
| 5.5 | Little or no training to operate the product | ||
| 5.6 | The ability to document and store the data from the procedure | ||
| 5.7 | A clear/crisp image | ||
| 5.8 | The ability to bookmark interesting areas | ||
| 5.9 | IVUS Patient information be available from the GE system |
| 5.1.1 | System will support combination tomographic and sagittal views. |
| o | Tomographic IVUS image. | ||
| o | Sagittal view vertically simultaneous with tomographic display. | ||
| o | All measurements need to be displayed on imaging views. | ||
| o | Data capture and display: record video loops and still images | ||
| o | Ability to replay or review captured images Data capture and display | ||
| o | Record video loops and still images |
| 5.1.2 | Display information: |
| o | Display in English | ||
| o | Volcano logo | ||
| o | Patient demographic information | ||
| o | Current date, time, software version. | ||
| o | Patient co-morbidity data available in the patient screen. |
| 5.1.3 | Measurements: distances, areas, longitudinal distances, and borders. |
| 5.1.4 | Support all current Volcano Cardiovascular and peripheral IVUS catheters and will have the ability and flexibility to support new catheter designs. |
| 5.1.5 | Standard local XGA video output will be provided, also 2 remote video outputs. | ||
| 5.1.6 | Output for network (DICOM), Ethernet RJ-45 connector | ||
| 5.1.7 | Will use standard Patient interface Module for phased array catheters, located within 30 feet of CPU | ||
| 5.1.8 | Connector on CPU chassis to allow the Volcano control panel, trackball and keyboard | ||
| 5.1.9 | System will support communication with the Volcano remote archiving station for color image printing and DVD recording of patient data and the PACS system (optional) |
Page 3 of 8
|
s5i-GE, Marketing Design | |||
| Input | ||||
| Control Number:806105-002/001 |
| 5.2.1. | System will have all computer components and PCBs integrated into a small, reduced weight, housing, suitable for mounting near the patient table in the Cath Lab and within 30 feet of the patient in the Control Room or Equipment Room. | ||
| 5.2.2. | A control panel, derived from the s5 will be mounted from the rails on the patient table in the Cath Lab and/or the Control Room and connect to the CPU by a single cable. It should also be provided with mounting hardware to mount it from a roll around equipment cart or from a wall. It should have suitable rubber feet so that it can be placed on a table top without marring the finish. | ||
| 5.2.3. | Approx. CPU dimensions: 16” X 16” X 6”(H x W x D). | ||
| 5.2.4. | Approx. CPU Weight: < 30 lbs. | ||
| 5.2.5. | Approx. Control Panel Dimensions: 10” X 16” x 4” (H x W x D.) | ||
| 5.2.6. | Approx. Control Panel Weight <10 Ibs. | ||
| 5.2.7. | The CPU must be capable of being attached to patient table. The mounting system must be designed for easy installation by a field service tech. | ||
| 5.2.8. | The Control Panel enclosure must be attachable to a boom/arm/mount that can be mounted on or to the control area of the patient table. It should also be capable of being mounted to a roll around IV pole cart. It should also be possible to place it on a flat table for desktop use. |
| 5.2.9. | One monitor on the GE monitor boom will be the IVUS monitor | ||
| 5.2.10 | Volcano Custom OEM Flat Panel Monitor: nominally 19“diagonal display area. (optional) | ||
| 5..2.10.1 Monitor screen visible in reduced light cath lab environment with minimal distortion when viewed from side or off angle (up to 30 degrees off angle). | |||
| 5.2.10.2. Contrast & brightness and image vertical size adjustment capability required. | |||
| 5.2.10.3. The display will be mounted on an articulated arm that allows the display position to be changed from stowed under the patient table, to easily visible at the patient table. | |||
| 5.2.10.4. The display should be provided with a table mount for remote display locations. |
| 5.2.12 | The Control Panel will connect to the CPU and allow Operation of the s5i-GE GUI and patient data entry using the pull out keyboard. The Control Panel can be mounted on or near the patient table, on a freestanding mobile IV pole carrier, or from an articulated wall mounted arm, depending on customer preferences. |
Page 4 of 8
|
s5i-GE, Marketing Design | |||
| Input | ||||
| Control Number: 806105-002/001 |
| 5.2.13 | The IVUS controls are integrated with the GE Innova touch screen, which is in the sterile field, and have the menus on the touch screen. The menus are under the IVUS tab and have several buttons replicating functionality of the Volcano control panel. The buttons can be enabled when the s5 receives the new patient message from Innova. Otherwise, these buttons are disabled. IVUS Tab selection exposes the IVUS buttons on the touch screen. The buttons on the Innova touch screen that are mapped to the control panel are as follows: |
| o | Record | ||
| o | Stop | ||
| o | Save Frame | ||
| o | Book ▇▇▇▇ | ||
| o | Play — plays the last acquired pullback loop | ||
| o | Ringdown | ||
| o | Pull Back — speed input | ||
| o | ChromaFlo | ||
| o | |||
| o | Measurements | ||
| o | End case |
| 5.2.13 | A remote archiving station will be provided to permit file transfer from the Cath Lab integrated CPU and burning patient files to a DVD from either a USB flash drive or a direct Ethernet connection to the CPU. It will be located in the control room. | ||
| 5.2.14 | Ability to send (push) DICOM images to the GE PACS server | ||
| 5.2.15 | Ability to review images on the CA-1000 |
| 5.3.1. | Will offer an intuitive, easy to use interface with minimal menus and minimal levels of operation. | ||
| 5.3.2. | Primary operator control will be through the Innova Touch screen | ||
| 5.3.3. | Additional controls, for measurement or dialog boxes which will include a trackball in the control panel | ||
| 5.3.4. | Selectable font size on the user interface |
Page 5 of 8
|
s5i-GE, Marketing Design | |||
| Input | ||||
| Control Number: 806105-002/001 |
| 5.4.1 | Application is always on. | ||
| 5.4.2 | The system is ready to go imaging in less than 120 seconds. The software and hardware will be properly initialized and calibrated during this time. |
| 5.5.1 | Innova touch screen design will follow the clinical workflow as designed on the Volcano control panel, which includes the Volcano icons | ||
| 5.5.2 | On screen step by step (“wizard”) available |
| 5.6.1 | Primary Storage Medium: Internal CPU hard drive(s). | ||
| 5.6.2 | Secondary Storage: Media will be DVD-R disk for archival purposes. | ||
| 5.6.3 | Tertiary Storage: Hospital DICOM server via the PACS network. | ||
| 5.6.4 | Ability to review data on systems from both primary and secondary sources. | ||
| 5.6.5 | Ability to view Volcano images on the CA-1000 | ||
| 5.6.6 | Will not support legacy Volcano CD-R IVUS disks | ||
| 5.6.7 | Ability to create a report reflecting the serial number of the catheters that have been used with this system. |
| 5.6.8 | A remotely located archiving station will be provided that connects to the CPU by means of a |
Page 6 of 8
|
s5i-GE, Marketing Design | |||
| Input | ||||
| Control Number:806105-002/001 |
| 5.6.9 | The archiving station allows the user to print and save to a DVD |
| 5.7.1 | Image Quality: s5i-GE system will have grayscale IVUS imaging quality equivalent to or better than current IVG Imaging System | ||
| 5.7.2 | Ability to adjust the live image. |
| 5.8.1 | Mechanical button on the console labeled bookmark | ||
| 5.8.2 | Button on the touch screen labeled bookmark | ||
| 5.8.2 | Mechanical button sends a software command to place a ▇▇▇▇ on the ILD. | ||
| 5.8.3 | The ▇▇▇▇ will be archived and available on the archived case. |
| 5.9.1 | Patient information appears on the IVUS screen and IVUS archived record | ||
| 5.9.2 | Ability to view the images on the GE CA 1000 | ||
| 5.9.3 | Ability to send (push) DICOM images to the GE PACS server |
Page 7 of 8
|
s5i-GE, Marketing Design | |||
| Input | ||||
| Control Number: 806105-002/001 |
| § | Hardware operators/“Button-pushers” |
| o | RN’s | ||
| o | CV technicians | ||
| o | Volcano field personnel |
| § | Decision makers/Data interpreters |
| o | Physicians |
GE will install the system and wiring.
Page 8 of 8
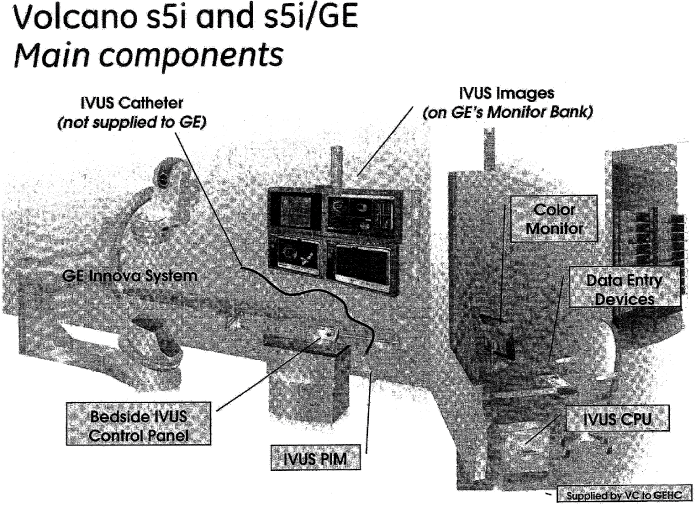
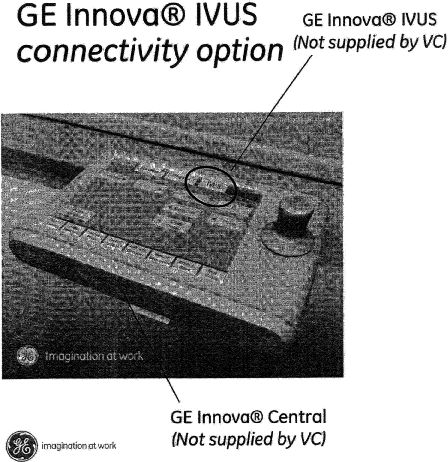
Purchase Specifications — Factory Acceptance Test
Spare parts Pricing Schedules and Lead-times
| Reference | Description | Leadtime in Weeks | Unit Price in Euros | |||
GE Integrity Policies
| a. | prohibition of illegal payments, | ||
| b. | political contributions, | ||
| c. | money laundering. |
GEHC Standard Terms and Conditions of Purchase
the Agreement shall prevail
Product Change Notice Form
| Before using this document, assure it is the latest revision. | ||
| See the Quality Systems Web page for current revision. | ||
 GLOBAL
SOURCING PROCEDURES GLOBAL
SOURCING PROCEDURES
|
▇▇▇▇://▇.▇▇▇.▇▇▇.▇▇▇/▇▇▇_▇▇▇/▇▇▇/▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇
▇.▇▇?▇▇▇▇▇▇▇ If you need assistance consult with your Mfg. Quality Representative |
|
GE Medical Systems
|
TITLE: Supplier Change Notice Control
|
PROCEDURE NO: 7.4.2-02GQP | |
OWNER: GSC EHS and Quality Compliance Manager
|
PAGE 1 of 4 | |
DATE ISSUED: 5/2004
|
DUE FOR REVIEW: 5/2006 |
| I. | SCOPE | |
| The purpose of this document is to define the criteria for initiating a Supplier Change Notice and the processes to be used to communicate and implement these change notices within GEMS and with the suppliers. | ||
| II. | REFERENCES | |
| 3.0.0-01GQP Terms and Definitions 7.4.2-02F Supplier Internal Transfer Risk Assessment Tool |
||
| III. | RESPONSIBILITIES |
| A. | Supplier Quality or equivalent is responsible for the following: |
| 1. | Act as the focal point for all generated SCN’s; Supplier or GEMS generated. | ||
| 2. | Assure SCN clarity, correct change categorization, and provide initial technical screening upon receipt. | ||
| 3. | Utilize the SCN Support Central process (SCN number assigned by Support Central). | ||
| 4. | Drive the completion of SCN’s via interface with modality point of contact. | ||
| 5. | Close the SCN upon completion. | ||
| 6. | Notify Supplier of SCN decision (Accept/Reject). | ||
| 7. | Ensure the master copy of the SCN is maintained in Support Central. |
| B. | Manufacturing Process Engineer or equivalent is responsible for the following: |
| 1. | Provide the manufacturing quality evaluation for the proposed change. | ||
| 2. | Provide leadership in review of requested change to insure process compatibility. | ||
| 3. | Respond to the SCN request in a timely fashion. | ||
| 4. | Consult with Engineering and other technical resources to determine required action. | ||
| 5. | Provide rework instruction for Manufacturing in the event of needed floor action. | ||
| 6. | Track SCN implementation at GEMS when a change requires manufacturing action. Communicate change requirement to Production Planning. |
| C. | Engineering is responsible for the following: |
| 1. | Provide technical evaluation of SCN requests. Consult with other modalities or P & L’s in the event of multi-modality or P & L use to determine impact and required action. | ||
| 2. | Act as the modality point-of-contact to review and approve the eSCN from Support Central site. | ||
| 3. | Notify the Manufacturing Process Engineer (MPE) or equivalent at respective modalities to determine the plan needed to properly analyze and approve the SCN. | ||
| 4. | Provide UL/ETL/CSA/FDA or other applicable agencies impact information as it related to the SCN if needed. | ||
| 5. | Respond to the SCN request in a timely fashion. |
| Before using this document, assure it is the latest revision. | ||
| See the Quality Systems Web page for current revision. | ||
 GLOBAL SOURCING PROCEDURES GLOBAL SOURCING PROCEDURES
|
▇▇▇▇://▇.▇▇▇.▇▇▇.▇▇▇/▇▇▇_▇▇▇/▇▇▇/▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇
▇.▇▇?▇▇▇▇▇▇▇ If you need assistance consult with your Mfg. Quality Representative |
|
GE Medical Systems
|
TITLE: Supplier Change Notice Control
|
PROCEDURE NO: 7.4.2-02GQP | ||
OWNER: GSC EHS and Quality Compliance Manager
|
PAGE 2 of 4 | ||
DATE ISSUED: 5/2004
|
DUE FOR REVIEW: 5/2006 | ||
| 6. | Determine if a Product Change Notice (PCN/ECR/ECO) is required, and if so, initiate the Change Notice. |
| D. | Supplier is responsible for the following: |
| 1. | Follow the SCN Procedure to communicate product changes to GEMS. | ||
| 2. | Manage product changes from supplier sources in a similar fashion. | ||
| 3. | Provide GEMS with adequate 90-day notice before implementation of changes. |
| IV. | PROCEDURE |
| A. | An SCN must be submitted for approval to GEMS for any change in the supplier’s product or the production process, including manufacturing location changes, that may affect the form, fit, function, reliability, serviceability, performance, safety, interchangability, regulatory compliance or interface with GEMS product. | ||
| B. | If the communication of the change comes from a GEMS affiliate, this change must not be documented in the SCN process. This change needs to be communicated to the appropriate modality responsible for the product so that the change can be handled through the internal change control process. | ||
| C. | If the supplier is building to GEMS prints/specifications, any changes to the supplier’s processes must be communicated to GEMS as a Supplier Change Notice. | ||
| D. | For SCN’s related to the change in manufacturing location, the SQE or equivalent must complete the Supplier Internal Transfer Risk Assessment Tool, 7.4.2-02F, as a guideline to decide if the transfer should be completed through the eNPl process. Instructions on use of the tool and guidelines for scoring are located in 7.4.2-02F. | ||
| E. | With regard to product changes, if GEMS owns the design and the supplier is building to print/specification, a Product Change Notice (PCN/ECR/ECO) is required to make changes. The supplier will use the SCN process to notify GEMS of these change requests and GEMS will be able to document this request and route it to the proper Engineering Design Owner. If the supplier owns the design, any changes to the design and the manufacturing process are required to be communicated to GEMS as a Supplier Change Notice. | ||
| F. | Once the SCN is determined to be needed, the supplier must communicate all required information to GEMS Sourcing Point of Contact (SCN Incoming Admin) a minimum of 90 days prior to the implementation of the change. The required information is noted by a red asterisk in the Support Central form but must include the following items: |
| 1. | All required supplier contact information per Support Central form. | ||
| 2. | GEMS part number(s) affected by the change. | ||
| 3. | Modality impacted by the change. | ||
| 4. | Desired cut-in date. | ||
| 5. | Is the part being transferred to another location. | ||
| 6. | Problem description followed by questions related to part use. | ||
| 7. | Description of the solution/change. |
| Before using this document, assure it is the latest revision. | ||
| See the Quality Systems Web page for current revision. | ||
 GLOBAL SOURCING PROCEDURES GLOBAL SOURCING PROCEDURES
|
▇▇▇▇://▇.▇▇▇.▇▇▇.▇▇▇/▇▇▇_▇▇▇/▇▇▇/▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇
▇.▇▇?▇▇▇▇▇▇▇ If you need assistance consult with your Mfg. Quality Representative |
|
GE Medical Systems
|
TITLE: Supplier Change Notice Control
|
PROCEDURE NO: 7.4.2-02GQP | ||
OWNER: GSC EHS and Quality Compliance Manager
|
PAGE 3 of 4 | ||
DATE ISSUED: 5/2004
|
DUE FOR REVIEW: 5/2006 |
| G. | The GEMS Sourcing Point of Contact will assign the appropriate SQE or equivalent to the SCN based on the Purchased Family of the affected part. | ||
| H. | The responsible Supplier Quality Engineer (SQE) or equivalent will ensure that the SCN form is properly and accurately filled out. |
| 1. | If the part is being transferred to another location, the SQE or equivalent must answer all of the questions related to location in the form to determine if an eNPI project must be initiated. | ||
| 2. | Based on the Problem Description and the Solution/Change, the SQE or equivalent must ensure that the questions related to part function and regulatory concerns have been addressed. | ||
| 3. | Once this information is accurate, the SQE or equivalent can approve the SCN completely if the SCN is deemed to have no known impact to GEMS product. | ||
| 4. | If the SCN is suspected to have an impact on GEMS product, the SQE or equivalent will select the route option and the SCN will move through the predetermined workflow. |
| I. | The Global Sourcing Leader (GSL), assigned in the workflow by the Purchased Family designation, will check the SCN for cost impact and will assign the Modality Point of Contact for the next workflow step. | ||
| J. | The Modality Point of Contact will review the part information and assign the Engineering Design Owner for the next step in the workflow. The Modality Point of Contact is also responsible tor expediting the SCN through the EDO process. | ||
| K. | The Engineering Design Owner will coordinate the change with all of the other appropriate functions to ensure that the change impact will be properly reviewed, evaluated and approved. |
| 1. | If the change is determined to have no impact on GEMS product, the SCN will be approved and routed to the Sourcing Point of Contact for closure. | ||
| 2. | If the change needs more information, such as samples, description of problem or clarification of solution, the SCN will be updated with the requests for information and routed back to the Sourcing Point of Contact. This request will be routed to the requestor/supplier for the requested information. |
| a. | When the required information has been gathered, the requestor/supplier will approve/forward the SCN to the Sourcing Point of Contact. | ||
| b. | The Sourcing Point of Contact will execute the workflow as per Step G above. |
| 3. | If the change is rejected, the SCN will be routed back to the Sourcing Point of Contact for communication and closure to the supplier as rejected. |
| L. | The SCN documentation including any attachment will be stored in Support Central for the life of the product as defined by GEMS. |
| V. | COUNSEL | |
| Sourcing Regulatory Leader |
| Before using this document, assure it is the latest revision. | ||
| See the Quality Systems Web page for current revision. | ||
 GLOBAL SOURCING PROCEDURES GLOBAL SOURCING PROCEDURES
|
▇▇▇▇://▇.▇▇▇.▇▇▇.▇▇▇/▇▇▇_▇▇▇/▇▇▇/▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇
▇.▇▇?▇▇▇▇▇▇▇ If you need assistance consult with your Mfg. Quality Representative. |
|
GE
Medical Systems
|
TITLE: Supplier Change Notice Control
|
PROCEDURE NO: 7.4.2-02GQP | |
OWNER: GSC EHS and Quality Compliance Manager
|
PAGE 4 of 4 | |
DATE ISSUED: 5/2004
|
DUE FOR REVIEW: 5/2006 | |
| Issue | ||||||||||
| Date | AUTHOR | APPROVAL | REASON FOR CHANGE | |||||||
| NAME | DATE | SIGNATURE | DATE | |||||||
10/03
|
K. Adam | 10/03 | ▇. ▇▇▇▇▇ | 10/03 | New Procedure | |||||
5/04
|
K. Adam | 5/04 | ▇. ▇▇▇▇▇ | 5/04 | Revised procedure to add the use of the eSCN Support Central tool and the use of the Transfer Risk Assessment form. | |||||
Repair Information and Service Packaging
 GEMS Europe GEMS Europe
|
EPO15R1 | |||
| TEST, PACKAGING and | ||||
| MARKING PROCEDURE | ||||
| FOR SERVICE PARTS | Revision 5 | |||
Global Parts Europe
|
Date : Oct 2002 |
| Revision | Date | Reason for change | ||
0
|
April 2nd 1993 | Initial release of PGR 15.1 | ||
1
|
October 8 1993 | Modification of pages 2,4,5,6 of the PGR 1.5.1 | ||
2
|
December 1994 | Renumbering | ||
3
|
January 1996 | Modification page 3: addition of quality label | ||
4
|
December 1996 | Update | ||
5
|
Oct 2002 | Redefine Packaging Guidelines and Quality Seal | ||
1.
|
PURPOSE OF PROCEDURE | 2 | ||||
2.
|
SCOPE | 2 | ||||
3.
|
TEST SPECIFICATIONS | 2 | ||||
4.
|
GENERAL PACKAGING SPECIFICATIONS | 2 | ||||
5.
|
CATEGORIES OF PACKAGING | 3 | ||||
6.
|
PACKAGE IDENTIFICATION | 4 | ||||
7.
|
QUALITY SEAL | 4 | ||||
8.
|
PACKING LIST | 4 | ||||
9.
|
USE OF GE LOGO | 4 | ||||
| APPENDIX 1 CIRCUIT BOARD PACKAGING GUIDELINES | 5 | |||||
| APPENDIX 2 EXAMPLES OF PACKAGING | 6 | |||||
| APPENDIX 3 LABELS USED | 7 | |||||
| APPENDIX 4 HOW TO SEAL A PACKAGING | 8 | |||||
Owner:
|
▇. ▇▇▇▇▇▇ | |
Function:
|
Quality Leader | |
Approved by:
|
▇. ▇▇▇▇▇ | |
Function:
|
Repair Operation and Development, Manager |
This document is proprietary to GE Medical Systems.
 GEMS Europe GEMS Europe
|
EPO15R1 | |||
| TEST, PACKAGING and | ||||
| MARKING PROCEDURE | ||||
| FOR SERVICE PARTS | Revision 5 | |||
Global Parts Europe
|
Date : Oct 2002 |
| 1. | PURPOSE OF PROCEDURE | |
| This procedure is to: | ||
| Define test, packaging and marking rules for service parts delivered by European suppliers when no other specific document is associated to a part. | ||
| Reference documents | ||
| GEMS Global Packaging Guideline recommendations # 2155696PRE (available on www.gegsn .com) | ||
| 2. | SCOPE | |
| This procedure applies to all service parts supplied to Service, both new and repaired parts when no other specific document is associated with the parts. This includes such items as electronic boards, HV supplies, power supplies, cables, components, and consumable and repairable accessories, without any restriction. | ||
| 3. | TEST SPECIFICATIONS | |
| Every part must be verified according to the characteristics provided by the manufacturer. Every part must be functionally verified according to current state of the art practices. | ||
| 4. | GENERAL PACKAGING SPECIFICATIONS |
| a. | Parts shall be individually packaged, even for small parts excep when specific instructions refer to a kit. | ||
| b. | Size of Package shall be adapted to the size of the part: as small as possible, while maintaining adequate protection. | ||
| c. | Weight restrictions — Units over 25 kg shall be banded to a pallet and they shall have special handling hazards marked on the outside, using international symbols. | ||
| d. | Hazardous materials shall be handled in the following manner: Package, ▇▇▇▇, label and document any material or product that is regulated as hazardous | ||
| e. | Packages shall provide basic protection from moisture, crushing, paint scuffing, corrosion, temperature and other product specific needs as required. |
This document is proprietary to GE Medical Systems.
Page 2/8
 GEMS Europe GEMS Europe
|
EPO15R1 | |||
| TEST, PACKAGING and | ||||
| MARKING PROCEDURE | ||||
| FOR SERVICE PARTS | Revision 5 | |||
Global Parts Europe
|
Date : Oct 2002 |
| f. | Repairable parts : Repairable parts must be wrapped in packages which facilitate their return to repair centers. The packaging must be replaced after each repair to obtain a clean package ready for the next shipment except if the package is considered as a Reusable package (see next paragraph). | ||
| g. | Reusable packaging: Reusable package may be used for expensive packaging. The main and expensive parts of the package can be reusable. This can be achieved easily by using a low-cost, replaceable external case. The external packaging must be replaced after each delivery to obtain a clean package ready for the next shipment. | ||
| h. | Protection from static electricity : All electronic boards and parts containing sensitive, accessible components must be protected from damage due to electrostatic discharge (ESD). ESD sensitive parts shall be wrapped in a conductive antistatic bag or film. The bag must be closed using a tamper-proof label (see Appendix 2 & 3). See Appendix 1 for detailed instructions about ESD. |
| 5. | CATAGORIES OF PACKAGING | |
| The following categories were created to give examples of the minimum necessary requirements. Components may still need specially designed packaging to be able to meet the testing standards. |
| a. | Electronic Components (circuit boards, etc) ESD bag with ESD foam individually boxed. The component shall fit snug in the box. Foam must be on all sides of the box. See Appendix 1 for specific guidelines |
||
| b. | Cables Individually packaged in a bag with connectors bubble wrapped. The bags shall be labelled. |
||
| c. | Mechanical/Plastic components Individually boxed and packaged in bubble wrap and/or foam. |
||
| d. | Batteries Shall be treated as a hazardous material and shall meet all government regulations unless otherwise stated as exempt. |
||
| e. | Power Supplies/Transformers Flexible foam shall be used to protect the units. Double cardboard shall be used. |
||
| f. | Pressurized Cans Shall be treated as a hazardous material and shall meet all government regulations, unless otherwise stated as exempt. |
||
| g. | Oversized items See specification 2155696PRE. |
This document is proprietary to GE Medical Systems.
Page 3/8
 GEMS Europe GEMS Europe
|
EPO15R1 | |||
| TEST, PACKAGING and | ||||
| MARKING PROCEDURE | ||||
| FOR SERVICE PARTS | Revision 5 | |||
Global Parts Europe
|
Date : Oct 2002 |
| h. | Computers/monitors Packaged with foam inserts in a corrugated container with access holes. |
||
| i. | Kits Packaged in a bag. The bags must be labelled. |
| 6. | PACKAGE IDENTIFICATION (SEE APPENDIX 3) | |
| All individual packages must be labelled with a label that specifies the following: |
| Ø | GEMS Part Number | ||
| Ø | Part Description | ||
| Ø | Bar Code encoding Part Number | ||
| Ø | Supplier name | ||
| Ø | Country of Origin | ||
| Ø | Purchase Order or Repair Order | ||
| Ø | Serial Number if required by GELS |
| The medium to be used shall be white adhesive labels with printing black ink. The size of the characters printed on the label must be greater than 6mm. | ||
| Labels must be placed in a visible location where they are protected from being turn off during handling. | ||
| 7. | QUALITY SEAL (SEE APPENDIX 3) | |
| Quality Seal is used as a seal of quality and placed on the external packaging (see Appendix 4). Quality Seals must be placed on all entry points (generally up and down). This assures the recipient that the package has not been opened in transit. However, should the package be opened to allow a check, the operator will have to repack and reseal the package with a properly filled up label identifying the warehouse. | ||
| 8. | PACKING LIST | |
| Packing List For “Service Parts” : Include a packing list that identifies the GEMS Part Number, PO number or Repair order number and quantities all of the materials included with the shipment. Any documentation needed to receive the part at the distribution facility must be on the outside of the package so opening is not required. |
||
| 9. | USE OF GE LOGO | |
| Use the GE logo on supplier packages only when specifically stated in the purchase order package. When used, specific guidelines must be followed for placement and size of all logo graphics. Specific layout instructions and artwork are available through GEMS on request. |
This document is proprietary to GE Medical Systems.
Page 4/8
 GEMS Europe GEMS Europe
|
EPO15R1 | |||
| TEST, PACKAGING and | ||||
| MARKING PROCEDURE | ||||
| FOR SERVICE PARTS | Revisions 5 | |||
Global Parts Europe
|
Date : Oct ▇▇▇▇ |
This provides a guarantee that a true faraday cage has been created around the board which is thus protected against static electricity and a means of determining whether or not the board has been removed from its protective envelop.
Ÿ Pre-printed cardboard case accommodating the marking labels
The package must however, hold the part firmly.
The case is preferably pre-printed
The package carries the mandatory marking labels described in Appendix 3.
This document is proprietary to GE Medical Systems.
Page 5/8
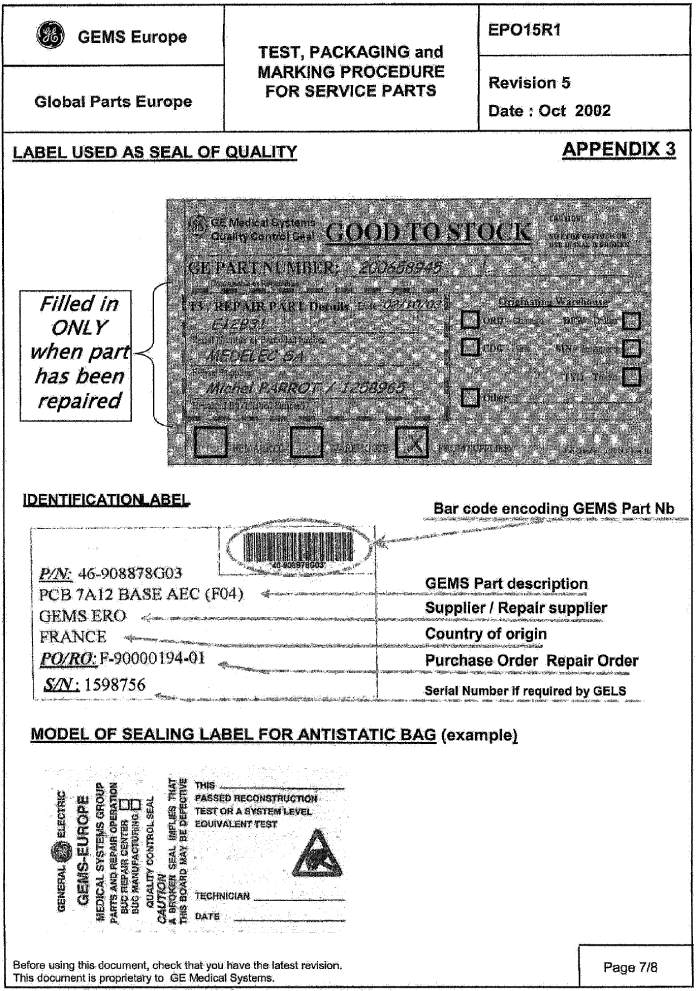
| Before using this document, assure it is the latest revision. | 7.4.2-01F-GQP | |
| See eLibrary DOC0079550 for current revision. | Revision: 01 | |
| Due for Review: 10/2007 |
__________________________________________________
__________________________________________________ _________________________________________________ _
| Before using this document, assure it is the latest revision. | 7.4.2-01F-GQP | |
| See eLibrary DOC0079550 for current revision. | Revisions: 01 | |
| Due for Review: 10/2007 |
Before
using this document, assure it is the latest revision.
|
7.4.2-01F-GQP | |
See
eLibrary DOC0079550 for current revision.
|
Revision: 01 | |
| Due for Review: 10/2007 |
Signature: Printed Name: |
/s/ ▇▇▇▇▇ ▇▇▇▇▇▇
|
|||
Title:
|
Director of Quality Assurance | |||
Date:
|
December 1, 2005 | |||
Supplier Name:
|
Volcano Corporation | |||
| Agreed to and Accepted by GE Healthcare | ||||
Signature:
|
/s/ ▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇ | |||
Printed Name:
|
▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇ | |||
Title:
|
Global Modality Sourcing Leader Ies. | |||
| Date: | December 1, 2005 | |||
Page 3 of 3
Initial Orders for Pilots
| Item | Qty | Description | Delivery Date | Total USD | ||||||
1
|
1 | s5i Pilot #1 | April 1, 2006 | USD [CONFIDENTIAL] | ||||||
2
|
1 | s5i Pilot #2 | April 7, 2006 | USD [CONFIDENTIAL] | ||||||
3
|
1 | s5i Pilot #3 | April 7, 2006 | USD [CONFIDENTIAL] | ||||||
4
|
1 | s5i Pilot #4 | April 7, 2006 | USD [CONFIDENTIAL] | ||||||
5
|
1 | s5i/GE Pilot #1 | May 1, 2006 | USD [CONFIDENTIAL] | ||||||
6
|
1 | s5i/GE Pilot #2 | May 1, 2006 | USD [CONFIDENTIAL] | ||||||
7
|
1 | s5i/GE Pilot #3 | May 1, 2006 | USD [CONFIDENTIAL] | ||||||
8
|
1 | s5i/GE Pilot #4 | May 1, 2006 | USD [CONFIDENTIAL] | ||||||
| 8 | USD [CONFIDENTIAL] | |||||||||
Countries of the Territory that Currently Maintain Exclusive Distribution
Agreements with Volcano
EU Language Policy
| GLB CPF1037-001-EMEA Language Requirements | Page 1 of 11 |
| 1. | EMEA: Europe, Middle East, Africa, | ||
| 2. | Labeling: All written, printed or graphic matter on a medical device or any of its containers or wrappers accompanying a medical device and relating to its identification, technical description and use. | ||
| This excludes shipping documentation and promotional material. | |||
| This excludes such information necessary for configuration, installation and service which must be performed by trained and English speaking staff | |||
| 3. | Label: All written, printed or graphic matter upon the device or immediate containers or wrappers necessary for the safe use of the device | ||
| 4. | Secondary Language/Flexibility: The possibility of an exemption of mandatory countryspecific language requirements in order to use another language. | ||
| 5. | GUI = Graphical User Interface: Any textual information visible to the end user e.g. patient and end user specific on-screen information or context menue or on-screen help, print outs, keyboards etc. | ||
| This excludes such information necessary for configuration, installation and service which must be performed by trained and English speaking staff. | |||
| 6. | Device Evaluation = Milestone: External Evaluation within NPI process. The purpose of the evaluation is to obtain customer’s feedback about the functions and features of the medical equipment. The medical equipment used for this customer evaluation shall be CE marked and thus demonstrates that the used medical product complies with the relevant Essential Requirements of the MDD 93/42/EEC. This is NOT Clinical Investigation or Clinical Evaluation. | ||
| TBD = Not started or pending investigation into potential legal exemptions |
COMPETENT AUTHORITIES OR LOCAL QRS PERSONNEL
GLB CPF1037-001 EMEA Language Requirements
|
Page 2 of 11 |
| Secondary | ||||||||||||
| Graphical User | Safety related | Operator’s | Device | Languages or known | ||||||||
| Country | Labels | Interface | Information | Manual | Evaluation | Flexibility | ||||||
Austria
|
German | German | German | German | TBD | none | ||||||
Belgium
|
Dutch + French + German must be provided |
Dutch + French + German must be provided |
Dutch + French + German must be provided |
Dutch + French + German must be provided |
English | The legal
requirement under
Belgian law (Royal
Decree of 18 March
1999) is that : - for patients, the three national languages must be used; |
||||||
| - for professional users, the national language of the professional must be used. | ||||||||||||
| Exceptions to the latter are possible if the following conditions are met: | ||||||||||||
| - a written agreement is concluded between the professional user and the manufacturer, his agent or authorised representative, mentioning the reasons why the national language cannot be used; | ||||||||||||
| - the agreement does not violate the national laws on the protection of employees | ||||||||||||
| - the agreement must be signed by the parties and kept at the disposal of the authorities. | ||||||||||||
Cyprus
|
English | English | English | English | English | |||||||
Czech Republic |
Czech | Czech for Safety instructions and user instructions | Czech | Czech | TBD | none | ||||||
Denmark
|
Danish | Danish | Danish | Danish | Danish | none |
GLB CPF1037-001 EMEA Language Requirements
|
Page 3 of 11 |
| Secondary | ||||||||||||
| Graphical User | Safety related | Operator’s | Device | Languages or known | ||||||||
| Country | Labels | Interface | Information | Manual | Evaluation | Flexibility | ||||||
Estonia
|
Estonian | Estonian or English | Estonian | Estonian | TBD | Only English GUI | ||||||
Finland
|
Finnish AND Swedish; English only if information is not safety-related | Finnish AND Swedish; English only if information is not safety-related | Finnish AND Swedish |
Finnish AND Swedish; English only if information is not safety-related | English | English only for information which is not relevant to ensure the safe use of the device | ||||||
France
|
French | French, except all Installation and Configuration Guides solely used by software installers or administrators. It has to be sure that medical personnel operating the hardware on which such software is installed will NOT use this software. | French | French | French | none | ||||||
Germany
|
German | German | German | German | English | Exception possible “in justified cases”, but: | ||||||
| - The language used must be easily understandable to the user or | ||||||||||||
| - Other measures must guarantee that the useris |
| GLB CPF1037-001 EMEA Language Requirements | Page 4 of 11 | |
| Graphical User | Safety related | Operator’s | Device | Secondary Languages or | ||||||||
| Country | Labels | Interface | Information | Manual | Evaluation | known Flexibility | ||||||
-appropriately instructed. And: |
||||||||||||
All information relating to aspects of safety
must be available in German or in the language
of the user (if non-German) |
||||||||||||
Greece
|
Greek/MoH may exclude devices designated exclusively for professional use from Greek language: in this case English is mandatory | English | Greek | Greek | English | Only English GUI | ||||||
Hungary
|
Hungarian | Hungarian; English only if all terms are explained in the manual | Hungarian | Hungarian | TBD | Only English GUI if all terms are explained in the Hungarian manual | ||||||
Iceland
|
Icelandic | Icelandic | Icelandic | Icelandic | TBD | other languages for professional use | ||||||
Ireland
|
English | English | English | English | English | none | ||||||
Italy
|
Italian | Italian | Italian | Italian (no instructions are needed for medical devices Class I or IIa if they can be used safely without such instructions | Italian | none | ||||||
Latvia
|
Latvian | Latvian or English | Latvian | Latvian | TBD | Only English GUI |
| GLB CPF1037-001 EMEA Language Requirements | Page 5 of 11 | |
| Graphical User | Safety related | Operator’s | Device | Secondary Languages or | ||||||||
| Country | Labels | Interface | Information | Manual | Evaluation | known Flexibility | ||||||
Liechtenstein
|
German | German | German | German | TBD | none | ||||||
Lithuania
|
Lithuanian | Lithuanian or English |
Lithuanian | Lithuanian | TBD | Only English GUI | ||||||
Luxembourg
|
French + German must be provided |
French + German must be provided |
French + German must be provided |
French + German must be provided | When the device is intended for the exclusive use by professionals the labelling and wording may be in English | none | ||||||
Malta
|
Maltese or English |
Maltese or English |
Maltese or English |
Maltese or English | English | English | ||||||
Netherlands
|
Dutch | Dutch | Dutch | Dutch | English if the user is a professional and trained | Exception possible on case-by-case basis by the
Ministry of Health; main criteria:
• Special training on the use of the medical device
• Use of the device only by specialists
• Use of the device on a regular basis to acquire
routine (min. two or three times a week) |
||||||
Norway
|
Norwegian | Norwegian | Norwegian | Norwegian | English if the | For electro-medical equipment: | ||||||
| user is a professional and trained | The use of an alternative language on
electro-medical equipment is possible. Applications for a waiver must be sent to: Direktoratet for samfunnssikkerhet og beredskap — Enhet for elektriske produkter Postboks 2014 |
|||||||||||
| 3103 Tønsberg |
| GLB CPF1037-001 EMEA Language Requirements | Page 6 of 11 | |
| Graphical User | Safety related | Operator’s | Device | Secondary Languages or | ||||||||
| Country | Labels | Interface | Information | Manual | Evaluation | known Flexibility | ||||||
| No special instructions exist to submit an
application-Just write a rationale
explaining the reason/s why we deliver
the equipment with English. The
professional users of the equipment must
be fluent enough in the alternative
language. The Competent Authority will ask
for more relevant information if required. For non-electrical-medical equipment: English for professional use |
||||||||||||
Poland
|
Polish | Polish or English if displayed information is explained in + referred to the Polish manual AND staff is trained | Polish | Polish | TBD | Only English GUI | ||||||
Portugal
|
Portugese | English with Portugese translation in an attached document |
Portugese | Portugese | Portugese | Local law does not know exceptions; but negotiations with the authorities are recommended by local lawyer | ||||||
Spain
|
Spanish | Spanish | Spanish | Spanish | TBD | None — but it is worth negotiating with the competent authorities | ||||||
Slovakia
|
Slovakian | Slovakian or English | Slovakian | Slovakian | TBD | Only English GUI | ||||||
Slovenia
|
Slovenia or English |
Slovenia or English |
Slovenia or English |
Slovenia or English | Slovenia or English |
Only medical devices which are intended by the manufacturer to be used exclusively by professional |
| GLB CPF1037-001 EMEA Language Requirements | Page 7 of 11 | |
| Graphical User | Safety related | Operator’s | Device | Secondary Languages or | ||||||||
| Country | Labels | Interface | Information | Manual | Evaluation | known Flexibility | ||||||
| medical staff may be in English. | ||||||||||||
Sweden
|
Swedish | Swedish | Swedish | Swedish | See: Flexibility | Exemptions are only granted in a limited sense i.e. in individual cases, to protect the health of a person. An exemption is only granted on a temporarily basis. | ||||||
| Medical Products Agency and National Board of Health
and Welfare jointly review applications for exemption
from the rules and regulations governing Medical
Devices. (cf. LVFS 2001:5, 6§ pt.12 concerning Active
Implants; LVFS 2001:7, 6§ pt.12 concerning IVD
Devices or LVFS 2003:11, 4§ pt. 3 and 7§ pt. 12,
respectively, concerning general Medical Devices) The following information should be included in the application: |
||||||||||||
| 1)State which requirement/’s in the regulations exemption is applied for | ||||||||||||
| 2) Specify the full designation/denomination of the product | ||||||||||||
| 3) Describe the product and state its intended purpose | ||||||||||||
| 4) State why the product, despite divergences from the regulations, can be used safely for patient and users (give an account for the results from a structured risk analysis) | ||||||||||||
| 5) State which period of time exemption is applied for and when the instructions for use in Swedish will be available | ||||||||||||
| 6) State the number of product units applied for | ||||||||||||
| 7) State to whom the product will be delivered | ||||||||||||
| 8) State why the product is indispensable | ||||||||||||
| 9) Describe measures taken to find an approved alternative product | ||||||||||||
| 10) State what will happen with the product when exemption period expires |
||||||||||||
| 11) Assurance (on the need) from Head of Dept if other than applicant |
| GLB CPF1037-001 EMEA Language Requirements | Page 8 of 11 |
| Graphical User | Safety related | Operator’s | Device | Secondary Languages or | ||||||||
| Country | Labels | Interface | Information | Manual | Evaluation | known Flexibility | ||||||
| Note that the Manufacturer/Distributor and the Care Provider both have to file an application Applications may be submitted either separately or as a joint document. Points1-7 are primarily of concern to Manufacturers/Distributors, 8-11 to Care Providers. Send applications to Medical Products Agency, Medical devices, ▇.▇. ▇▇▇ ▇▇, ▇▇-▇▇▇ ▇▇ ▇▇▇▇▇▇▇, ▇▇▇▇▇▇ | ||||||||||||
▇▇▇▇▇▇▇▇▇▇▇
|
French + German + Italian must be provided |
French + German + Italian must be provided |
French + German + Italian must be provided |
French + German + Italian must be provided |
English: signed agreement with customer. It shall be ensured that customer understands English | According to article 7 of the Swiss Regulation on Medical Devices (▇▇▇.▇▇▇▇▇.▇▇/▇▇/▇/▇▇/▇▇▇ 124/a7.html ) any product information in the frame of the European Directive must be given in the three national languages, i.e. German, French and Italian. However, the competent Federal authority (Fachstelle Medizinprodukte, Bundesverwaltung Bern, ▇▇. ▇▇▇▇▇▇▇, Fax ▇▇▇ ▇▇▇▇▇ ▇▇ ▇▇) may make exceptions if (i) safety of patients, users and third parties is protected and (ii) compliance with the language requirements would cause a disproportionate effort. | ||||||
United Kingdom |
English | English | English | English | English | N/A |
| Page 9 of 11 |
| Graphical User | Safety–related | Operator’s | Device | Secondary Languages or | ||||||||
| Country | Labels | Interface | Informations | Manual | Evaluation | known flexibility | ||||||
Albania
|
Albanian or English | Albanian or English | Albanian or English | Albanian or English | English. | |||||||
Azerbeidjan
|
Russian or English | Russian or English | Russian or English | Russian or English | English | |||||||
Belarus
|
Russian or English | Russian or English | Russian or English | Russian or English | English | |||||||
Bosnia–Herzegovina
|
English | English | English | English | English | |||||||
Bulgaria
|
Bulgarian or English | Bulgarian or English | Bulgarian or English | Bulgarian or English | English | |||||||
Croatia
|
Croatian or English | Croatian or English | Croatian or English | Croatian or English | English | |||||||
Kasachstan
|
Russian or English | Russian or English | Russian or English | Russian or English | English | |||||||
Kyrgisia
|
Russian or English | Russian or English | Russian or English | Russian or English | English | |||||||
Macedonia
|
Serbian or English | Serbian or English | Serbian or English | Serbian or English | English | |||||||
Moldavia
|
Russian or English | Russian or English | Russian or English | Russian or English | English | |||||||
Romania
|
Romanian for all info necessary for the safe use | Romanian or English | Romanian for all information necessary for the safe use | Romanian for all information necessary for the safe use | Only English GUI | |||||||
Russia (CIS)
|
Russian | Russian or English | Russian | Russian | Only English GUI | |||||||
Tadjikistan
|
Russian or English | Russian or English | Russian or English | Russian of English | English | |||||||
Turkmenistan
|
Russian or English | Russian or English | Russian or English | Russian or English | English | |||||||
Ukraine
|
Russian or English | Russian or English | Russian or English | Russian or English | English | |||||||
Uzbekistan
|
Russian of English | Russian or English | Russian or English | Russian or English | English | |||||||
[ILLEGIBLE]
|
[ILLEGIBLE] | [ILLEGIBLE] | [ILLEGIBLE] | [ILLEGIBLE] | [ILLEGIBLE] |
| GLB CPF1037-001 EMEA Language Requirements | Page 10 of ▇▇ |
| Graphical User | Safety related | Device | ||||||||||
| Country | Labels | Interface | Informations | Operator’s Manual | Evaluation | Secondary Language | ||||||
Egypt
|
English | English | English | English | English | |||||||
Bahrain
|
English | English | English | English | English | |||||||
Iran
|
English | English | English | English | English | |||||||
Israel
|
English | English | English | English | English | |||||||
Jordan
|
English | English | English | English | English | |||||||
Kuwait
|
English | English | English | English | English | |||||||
Lebanon
|
French | French | French | French | English | |||||||
Oman
|
English | English | English | English | English | |||||||
Palestine
|
English | English | English | English | English | |||||||
Pakistan
|
English | English | English | English | English | |||||||
Quatar
|
English | English | English | English | English | |||||||
Saudi Arabia
|
English | English | English | English | English | |||||||
Syria
|
English | English | English | English | English | |||||||
Turkey
|
English | English | English | Turkish | Only English GUI | |||||||
United Arab Emirates
|
English | English | English | English | English |
| GLB CPF1037-001 EMEA Language Requirements | Page 11 of 11 |
| Graphical User | Safety related | Device | ||||||||||
| Country | Labels | Interface | Informations | Operator’s Manual | Evaluation | Secondary Language | ||||||
Algeria
|
French | French | French | French | French | |||||||
Ivory Coast
|
French | French | French | French | French | |||||||
Morocco
|
French | French | French | French | French | |||||||
Senegal
|
French | French | French | French | French | |||||||
South Africa
|
English | English | English | English | French | |||||||
▇▇▇▇▇▇▇
|
▇▇▇▇▇▇ | French | French | French | French |

