CLINICAL SERVICES MASTER AGREEMENT
| 1.1. | The specific responsibilities and obligations to be performed by Omnicare CR with respect to a Project (the “Services”), as set forth in the applicable Protocol(s), are expressly set forth in Exhibit(s) attached to this Master Agreement, which, together with the Exhibit(s) attached hereto, are incorporated by reference herein. No Exhibit will be attached to this Master Agreement or become effective without first being executed by duly authorized representatives of the parties hereto. To the extent any terms set forth in an Exhibit shall conflict with the terms set forth in this Master Agreement, the terms of this Master Agreement will take precedence unless the Exhibit expressly states that a conflicting term is intended to modify a specific term in this Master Agreement. The responsibility for the Services is being transferred to Omnicare CR in accordance with 21 C.F.R. §312.52. Those responsibilities and obligations not specifically transferred to and assumed by Omnicare CR in this Master Agreement or the Exhibit(s) as constituting part of the Services shall be and remain the sole responsibility of Sponsor. | ||
| 1.2. | Omnicare CR agrees that Omnicare CR will provide the Services in accordance with (a) all applicable federal laws and regulations, including standards of Good Clinical Practices; and (b) the standards and practices that are generally accepted in the industry and exercised by other persons engaged in performing similar services. | ||
| 1.3. | In the discharge of its duties, Omnicare CR shall comply with all reasonable directions of Sponsor as may be given in writing from time to time in respect of the Services. |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| 1.4. | Omnicare CR shall at all times provide sufficient appropriately-trained and qualified clinical research personnel on a given Project to meet the demands of said Project. | ||
| 1.5. | In the event that Sponsor or a third-party engaged by Sponsor performs an audit of the Project, Omnicare CR will respond in writing to the audit findings within thirty (30) days of receipt of same. | ||
| 1.6. | Omnicare CR shall use its commercially reasonable efforts, skills and abilities to promote the interests of Sponsor and to diligently and competently perform its duties under this Master Agreement. |
| 2. | Payment |
| 2.1. | In consideration of the Services, Sponsor shall pay to Omnicare CR: (a) the Service Fees (as defined in Section 2.2); and (b) the Pass-Through Costs (as defined in Section 2.3). | ||
| 2.2. | As used in this Master Agreement, the term “Service Fees” means all amounts due for the Services, exclusive of the Pass-Through Costs. The estimated Service Fees and the payment schedule therefor are set forth in the Exhibit(s), and shall be increased to include: (a) the costs of any additional Services required as a result of Project changes by Sponsor; (b) any costs that arise out of or relate to a Force Majeure as outlined in Section 10 below, and (c) where a Project requires more time than allotted in the Exhibit(s), and the parties agree to continue such Project beyond the expected conclusion date, any additional costs that may be incurred in order to complete such Project, at the contractual rates set forth in the applicable Exhibit(s). All payments of Service Fees shall be made within thirty (30) days of receipt of invoice. If any payment of Service Fees is late by more than thirty (30) days, such payment shall be subject to a liquidated damages fee of 1.5% per month of the outstanding balance. | ||
| 2.3. | As used in this Agreement, the term “Pass-Through Costs” means all investigator, Institutional Review Board or other applicable pass-through costs actually and reasonably incurred by Omnicare CR under this Agreement or the Exhibit(s) in order to expedite successful completion of a Project, which costs are normal and routine to studies similar to such Project (e.g., advancing an investigator’s Institutional Review Board fee and investigator grant payments or reimbursing reasonable additional, unbudgeted patient expenses). In order to enable Omnicare CR to maintain a balance to be applied towards investigator-related Pass-Through Costs, Omnicare CR shall invoice Sponsor for all reasonably anticipated Pass-Through Costs (the “Estimated Pass-Through Costs”) in advance of the expected payment date therefor. Except with respect to investigator-related payments, which are payable upon receipt, all Pass-Through Costs shall be paid within thirty (30) days of receipt of invoice. If any payment Pass-Through Costs is late by more than thirty (30) days, such payment shall be subject to liquidated damages of 1.5% per month of the outstanding balance. The anticipated Pass-Through Costs and Estimated Pass-Through Costs and the payment schedule therefor are set forth in the Exhibit(s). |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
2
| 2.4. | Where applicable, language pertaining to annual price increases will be included in each Exhibit. | ||
| 2.5. | Taxes (and any penalties thereon) imposed on any payment made by Sponsor to Omnicare CR will be the responsibility of Omnicare CR. Any sales tax, usage tax, Value Added Tax (VAT), or other similar taxes shall be the responsibility of the Sponsor. | ||
| 2.6. | Sponsor shall make payment direct to the following bank account nominated by Omnicare CR: |
First Union National Bank
Philadelphia, PA
ABA # ****
Acct # ****
| 3.1. | That certain Confidentiality Agreement by and between Omnicare CR and Sponsor dated as of 25th November 2004 (the “Confidentiality Agreement”) is hereby terminated and of no further force or effect. | ||
| 3.2. | In connection with the performance of the Services, Sponsor shall provide to Omnicare CR, and Omnicare CR shall have access to, Sponsor’s Confidential Information. As used in this Master Agreement, “Sponsor’s Confidential Information” means any (a) information provided by, or developed for, Sponsor within the framework of or in undertaking activity pursuant to this Master Agreement, the Exhibit(s) or the Confidentiality Agreement; or (b) data collected during a Project. | ||
| 3.3. | In connection with this Master Agreement, Sponsor will have access to, or become acquainted with, Omnicare CR’s Confidential Information. As used in this Master Agreement, “Omnicare CR’s Confidential Information” means any (a) information generated or obtained in connection with Omnicare CR’s pricing, proposals or contracts (including the provisions of this Master Agreement and the Exhibit(s)); (b) of Omnicare CR’s procedures, programs, guidelines or policies (including, without limitation, its Standard Operating Procedures); or (c) information designated in writing as “confidential.” | ||
| 3.4. | Neither Sponsor’s Confidential Information nor Omnicare CR’s Confidential Information (collectively, “Confidential Information”) shall include any information that: |
| (a) | was known by the receiving party at the time of disclosure to it by the disclosing party, or that is independently developed or discovered by the receiving party, after disclosure by the disclosing party, without the aid, application or use of any |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
3
| item of the disclosing party’s Confidential Information, as evidenced by written records; | |||
| (b) | is now or subsequently becomes, through no act or failure to act on the part of the receiving party, generally known or available; | ||
| (c) | is disclosed to the receiving party by a third party authorized to disclose it; or | ||
| (d) | is required by law or by court or administrative order to be disclosed; provided, that the receiving party shall have first given prompt notice to the other party of such required disclosure. |
| 3.5. | Each party shall exercise due care to prevent the unauthorized use or disclosure of the other party’s Confidential Information, and shall not, without the other party’s prior written consent, (a) use the other party’s Confidential Information for any purpose other than performing its obligations under this Master Agreement and the Exhibit(s); or (b) disclose or otherwise make available, directly or indirectly, any item of the other party’s Confidential Information to any person or entity other than those employees, independent contractors, agents or investigators of such party and/or its affiliate entities (collectively, “Representatives”) who reasonably need to know the same in the performance of such party’s obligations under this Master Agreement (including the Exhibit(s)), or in order to make decisions or render advice in connection therewith. For the convenience of the parties, each party acknowledges that unless precluded in writing by the other party, Confidential Information may be transmitted to a party and/or its Representatives via the Internet. Each party shall advise its Representatives who have access to the other party’s Confidential Information of the confidential nature thereof, and agrees that such Representatives will be bound by terms of confidentiality and restrictions on use with respect thereto that are at least as restrictive as the terms of this Section 3. | ||
| 3.6. | The provisions of this Section 3 shall survive for a period of five (5) years from the date of any expiration or termination of this Master Agreement, however caused. | ||
| 3.7 | Omnicare CR acknowledges that in accordance with Section 1043A of the Corporations Act of the Commonwealth of Australia: |
| (a) | Omnicare CR may from time to time as a consequence of the services provided hereunder possess confidential information which may have a material effect on the price or value of the securities of Sponsor and may therefore constitute “inside information” for the purposes of the Corporations Act; and | ||
| (b) | as an insider one must not (whether as principal or agent in possession of such inside information): |
| • | apply for, acquire or dispose of such securities, or enter into an agreement to apply for, acquire or dispose of any such securities; or | ||
| • | procure another person to apply for, acquire or dispose of, or to enter into an agreement to apply for acquire or dispose of, any such securities. |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
4
| and acknowledges further that: |
| (c) | once in possession of confidential information, a party may be subject to the xxxxxxx xxxxxxx restrictions imposed by the Corporations Act and may be prohibited from trading in the securities (as noted above) and / or communicating the confidential information to any other person who would be likely to subscribe for, purchase or sell securities, or procure a third person to do the same until such date when this confidential information has been made “public” in terms of the requirements of the Corporations Act; and | ||
| (d) | it must seek its own legal advice on its responsibilities under the Corporations Act and that neither party purports that the comments in this clause are either advice or a comprehensive description of the provisions of the Corporations Act. |
| 4.1. | All (a) of Sponsor’s Confidential Information (including, without limitation, all original Project records and reports), (b) unused clinical supplies provided by Sponsor, and (c) complete and incomplete Case Report Forms, which in any case are in Omnicare CR’s possession, shall be and remain Sponsor’s property; provided, however, that Omnicare CR may retain one copy of Sponsor’s Confidential Information in its files for archival purposes, as a means of determining any continuing obligations under this Master Agreement (including the Exhibit(s)). | ||
| 4.2. | All inventions, improvements in know-how, new uses, processes and compounds involving the study drug(s) and/or product(s) covered by this Master Agreement and/or the Exhibit(s) that are conceived or reduced to practice as a direct result of the Project(s) (“Inventions”) shall be and remain the sole property of Sponsor. Omnicare CR shall cooperate with Sponsor in obtaining, at Sponsor’s sole cost and expense, any patent protection as may be available for the Inventions, and shall execute all documents reasonably deemed necessary by Sponsor for purposes of procuring such patent protection. Omnicare CR agrees that Omnicare CR shall endeavor to ensure contractually the prompt disclosure to Sponsor by any investigator, employee or other individual retained by Omnicare CR for a Project of any Inventions, as well as the cooperation of such persons in securing patent protection as set forth herein. | ||
| 4.3. | Notwithstanding the foregoing, Sponsor acknowledges that Omnicare CR and their respective professional staff currently possess certain inventions, processes, know-how, trade secrets, methods, approaches, analyses, improvements, other intellectual properties and other assets including, but not limited to, clinical trial management analyses, analytical methods, procedures and techniques, computer technical expertise and proprietary software, and technical and conceptual expertise in the area of conducting clinical trials, all of which have been developed independently by Omnicare CR without the benefit of any information provided by Sponsor (collectively, “Omnicare CR Property”). Sponsor agrees that any Omnicare CR Property which is used, improved, modified or developed by Omnicare CR under or |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
5
| 7.1. | Sponsor’s authorized representatives may visit Omnicare CR’s site and facilities at reasonable times and with reasonable frequency during normal business hours and upon reasonable advance written notice, to observe the progress of any Services. All such visits shall be subject to Omnicare CR’s restrictions and procedures relating to safety, security and protection of Confidential Information, and in connection therewith, Sponsor’s authorized representatives may be required to sign a confidentiality agreement, or an access agreement for special access-controlled areas. | ||
| 7.2. | During the term of this Master Agreement, Omnicare CR shall maintain all materials and all other data obtained or generated by Omnicare CR in the course of providing the Services hereunder, including all computerized records and files. Omnicare CR shall cooperate with any reasonable internal review or audit by Sponsor and make available to Sponsor for examination and duplication, during normal working hours and at mutually agreeable times, all documentation, data and information relating to a Project. | ||
| 7.3. | Upon the expiration or termination of this Master Agreement, all materials and all other data and information obtained or generated by Omnicare CR as a direct result of providing the Services hereunder will, at Sponsor’s option and cost and expense, be (i) delivered to Sponsor’s offices at the address provided herein in such form as is then currently in the possession of Omnicare CR, (ii) retained by Omnicare CR for Sponsor for a period of two years, or (iii) disposed of as directed in writing by Sponsor, unless such materials are otherwise required to be stored or maintained by |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
6
| Omnicare CR under applicable law. In no event shall Omnicare CR dispose of any materials or data or other information obtained or generated by Omnicare CR as a direct result of providing the Services without first giving Sponsor sixty (60) days prior written notice of its intent to dispose same. Notwithstanding the foregoing, Omnicare CR may retain copies of any such materials, data and information as is reasonably necessary for regulatory purposes or to demonstrate the satisfaction of its obligations hereunder, all subject to the confidentiality obligations set forth herein. |
| 8.1. | Sponsor agrees to indemnify, defend and hold harmless Omnicare CR and its Affiliate Entities, and their respective officers, directors and employees from and against any and all claims, demands, investigations, suits or actions (each a “Claim”) for any and all liabilities, losses, damages, penalties, costs or expenses of every kind whatsoever (including but not limited to court costs, legal fees, awards of settlements) arising out of, in connection with or related to this Master Agreement and/or the Exhibit(s); provided, however, that Sponsor’s indemnity obligations under this Section 8 shall not apply to any Claim to the extent arising directly from Omnicare CR’s negligence or willful malfeasance. | ||
| 8.2. | Omnicare CR agrees to indemnify, defend and hold harmless Sponsor and its respective officers, directors and employees from and against any and all Claims for any and all liabilities, losses, damages, penalties, costs or expenses of every kind whatsoever (including but not limited to court costs, legal fees, awards or settlements) arising out of, in connection with or related to any breach of this Master Agreement and/or the Exhibit(s) by Omnicare CR, including any breach of warranties, or any willful, unlawful or negligent act or omission of Omnicare CR. | ||
| 8.3. | Each person or entity seeking indemnification under this Section 8 (the “Indemnified Party”) shall, as a condition thereto, notify the other party within twenty (20) days after the receipt of notice of the Claim; provided, however, that the other party shall not be released from its obligations under this Section 8 if the failure to notify that other party within twenty (20) days does not materially prejudice the defense of such Claim. That other party shall have the right to select defense counsel and to direct the defense or, with the consent of the indemnified party (which consent shall not be unreasonably withheld) settlement of, any Claim. In the event that representation of an Indemnified Party and the other party by the same counsel would be a conflict of interest for such counsel, the Indemnified Party may select its own independent counsel without relieving the other party of its obligations under this Section 8. Under no circumstances shall an Indemnified Party settle or otherwise compromise any Claim without the other party’s prior written consent. | ||
| 8.4. | This clause shall survive the expiration or termination of the Master Agreement and/or the Exhibit(s). |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
7
| 9.1. | Either party may terminate this Master Agreement and/or any Exhibit at any time and for any reason upon a minimum of thirty (30) days’ prior notice, provided that Omnicare CR may only terminate under this sub-clause in the event that there are no active Exhibits, ie, all tasks under all Exhibits have been completed. | ||
| 9.2. | Without limiting the generality of any other clause in this Master Agreement or the remedies available to either party, either party may terminate this Master Agreement immediately by notice in writing if: |
| (a) | the other party is in material breach of any terms of this Master Agreement or any Exhibit and, where such breach is remediable, such party fails to remedy such material breach or establish a corrective action plan to cure such material breach (which plan shall outline actions to be taken and relevant dates) acceptable to the non-breaching party within fifteen (15) days of receiving notice to do so from the non-breaching party; | ||
| (b) | the other party is in serious and/or presistent breach of any terms of this Master Agreement or any Exhibit. Failure to perform in accordance with the corrective action plan outlined above shall be deemed a serious breach; or | ||
| (c) | the other party becomes, threatens to become or is in jeopardy of becoming insolvent. |
| 9.3. | Upon receipt of any notice of termination, a party shall avoid or limit, to the extent practicable, incurring any futher commitments, obligations or costs which would otherwise result in Service Fees and Pass-Through Costs. | ||
| 9.4. | Upon any early termination, Sponsor shall pay to Omnicare CR all Service Fees and Pass-Through Costs due and owing based upon Services completed and costs incurred through the effective date of termination, including costs for materials and/or services previously acquired or contracted for which will not be used for the Services as a result of such termination. | ||
| 9.5. | Any funds held by Omnicare CR which by contract definition or amendment are deemed unearned (including, without limitation, any Estimated Pass-Through Costs not used to satisfy Pass-Through Costs) shall be returned to Sponsor within sixty (60) days after conclusion or termination of the Project(s) set forth in the applicable Exhibit(s). | ||
| 9.6. | Following completion or termination of any Project, Omnicare CR shall forward all original Project records and reports to Sponsor (or to a repository designated by Sponsor in writing) at Sponsor’s sole cost and expense. Thereafter, Omnicare CR shall retain any documentation related to such Project in compliance with Omnicare CR’s corporate policy on retention and destruction of records. |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
8
| 12.1. | Sponsor acknowledges that the results of the Project(s) are inherently uncertain and that, accordingly, there can be no assurance, representation or warranty by Omnicare CR that the study drug(s) and/or product(s) covered by this Master Agreement and/or the Exhibit(s) can, either during the term of this Master Agreement or thereafter, be developed sucessfully or, if so developed, will receive the required approval(s) from the FDA orther regulatory agency or authority. | ||
| 12.2 | Both parties acknowledge that the Services constitute research and development. Accordingly, Sponsor’s sole remedy for any breach or default hereof by Omnicare CR shall be termination of this Master Agreement or the applicable Exhibit as herein provided or a return of any Service Fees paid to Omnicare CR for Services improperly performed or not performed. In no event shall Omnicare CR be liable for any special, indirect, incidental or consequential damages (whether in contract or tort). |
| 13.1. | Omnicare CR represents and warrants that Omnicare CR has not been nor is currently: |
| (a) | an individual who has been debarred by the FDA pursuant to 21 U.S.C. §335a (a) or (b) (a “Debarred Individual”) from providing services in any capacity to a |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
9
| person that has an approved or pending drug product application, or an employer, employee or partner of a Debarred Individual; or | |||
| (b) | a corporation, partnership, or association that has been debarred by the FDA pursuant to 21 U.S.C. §335a (a) or (b) (a “Debarred Entity”) from submitting or assisting in the submission of an abbreviated new drug application, or an employee, partner, shareholder, member, subsidiary or affiliate entity of a Debarred Entity. |
| 13.2. | Omnicare CR further represents and warrants that Omnicare CR has no knowledge of any circumstances which may affect the accuracy of the representations and warranties set forth in Section 13.1 including, but not limited to, FDA investigations of, or debarment proceedings against, Omnicare CR or any person or entity performing, or rendering assistance related to, the Services. Omnicare CR will notify Sponsor promptly upon becoming aware of any such circumstances during the term of this Master Agreement. |
If to Omnicare CR:
|
Omnicare CR, Inc. | |
| 000 Xxxxxxxxx Xxxx | ||
| Xxxx xx Xxxxxxx, XX 00000 | ||
| Attention: Global Client Contracts | ||
If to Sponsor:
|
Xxxxxx Operations Pty Ltd | |
| Xxxxx 0, 0 Xxxxxxxxx Xxxxx Xxxx | ||
| Xxxxxxxx | ||
| XXX 0000 | ||
| Xxxxxxxxx |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
10
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
11
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
12
| Xxxxxx Operations Pty Ltd | Omnicare CR, Inc. | |||||||||
By:
|
/s/ Xxxxxxx Xxxxxxxx | By: | /s/ Xxxxx X. Xxxxx | |||||||
Name:
|
Xxxxxxx Xxxxxxxx | Name: | Xxxxx X. Xxxxx | |||||||
Title:
|
Director | Title: | Senior Vice President Global Marketing & Business Development |
|||||||
| APPROVED | ||||||||||
| LEGAL DEPT. | ||||||||||
| 29-July-05 | 28-July-05 | |||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
between Xxxxxx Operations Pty Ltd. and Omnicare CR, Inc.,
dated June 1, 2005.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 1
| Major Milestones | Projected Timeline | |
Study Start
|
**** | |
First Patient In
|
**** | |
Last Patient In
|
**** | |
Last Patient End of Treatment
|
**** | |
Last CRF to Data Management
|
**** | |
Database Close
|
**** | |
Statistical Analysis
|
**** | |
Study Close/Report
|
**** | |
Total Study Duration
|
**** |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 2
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 3
| • | Create all investigator agreement templates and patients budget template with the Sponsor | ||
| • | When possible, set up pre approved alternative language and budget parameters in anticipation of site negotiation | ||
| • | Negotiate all investigator agreements that satisfy Sponsor requirements on behalf of the Sponsor | ||
| • | Incorporate changes using pre-approved alternative parameters and if changes are outside parameters and secure approval by the Sponsor | ||
| • | Obtain signatures from investigators, institutions, facilities, site management organizations on all investigator agreements as needed | ||
| • | Collect IRS Form W9 and payment information sheets from sites | ||
| • | Track documentation and communication between the sites, Omnicare CR Project Management and the Sponsor. |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 4
| • | Therapeutic are and clinical development background | ||
| • | Protocol and CRF | ||
| • | Discussion of therapeutic implications for this study | ||
| • | Monitoring guidelines | ||
| • | Data handling rules from Data Management Plan |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 5
| • | Identify appropriate location(s) for the meeting | ||
| • | Negotiate, organize, and make hotel arrangements (e.g. meeting space and lodging) | ||
| • | Secure discounted travel arrangements and issue tickets to investigators and study coordinators | ||
| • | Prepare meeting materials (e.g. Welcome letters/packets, and name badges) | ||
| • | Arrange meal functions and off-site events | ||
| • | Manage administrative aspects associated with the meeting | ||
| • | Provide on-site assistance at the meeting (the coordinator will arrive at the location prior to the meeting and will stay after the meeting to manage any outstanding arrangements such as shipping meeting materials | ||
| • | Provide post meeting service including reconciliation of outstanding meeting bills |
| • | Assist in defining meeting requirements and outlining a meeting agenda | ||
| • | Preparing necessary meeting materials (e.g., annotated CRFs, presentation materials, etc.) | ||
| • | Coordinating presentations | ||
| • | Conducting presentations and/or workshops on protocol, CRF, study conduct issues, and reporting of serious adverse events |
| • | Pre study selection visits | ||
| • | Initiation visits | ||
| • | Interim monitoring visits | ||
| • | Close out visits |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 6
| • | Informed consent process | ||
| • | IRB/IEC approval | ||
| • | Ensuring proper AE and SAE reporting and documented follow up | ||
| • | Adherence the protocol | ||
| • | 100% source documentation verification and data query clarification | ||
| • | Investigational product administration and accountability | ||
| • | Protocol Compliance | ||
| • | Site training and support |
| • | Site staff qualifications and experience | ||
| • | Site staffing, facilities, storage and equipment | ||
| • | Accessibility and eligibility of subjects | ||
| • | Review and retrieval of regulatory documents (i.e. FDA 1572, protocol signature page, curricula vitae, medical licenses, certification of investigators’ financial disclosure, etc.) | ||
| • | Accurate and timely completion of all CRF and source data |
| • | Site Visit Report and follow-up letters | ||
| • | Providing site training and support through communications | ||
| • | Generation of status reports | ||
| • | Maintenance of tracking tools/logs | ||
| • | Facilitation of DCF resolution |
| • | Latest version of the Protocol for this study | ||
| • | Consent form process | ||
| • | AE and SAE reporting procedures and contact information |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 7
| • | Case report form completion and maintenance | ||
| • | Source documentation requirements | ||
| • | Drug accountability requirements |
| • | Principal investigator qualifications and experience | ||
| • | Site staffing, facilities, storage and equipment | ||
| • | Adequacy of and accessibility to subject population | ||
| • | Access to source documentation | ||
| • | Status of regulatory documentation (i.e. FDA 1572, protocol sign-off page, curricula vitae, medical licenses, certification of investigators’ financial disclosure, etc.) | ||
| • | IRB and ethics committee issues | ||
| • | Laboratory and pharmacy certifications and normal ranges (if applicable) | ||
| • | Investigator agreement and indemnification issues | ||
| • | Recommendations for investigational site approval or exclusion from the study |
| • | Study goals and obligations | ||
| • | Investigator brochure | ||
| • | Protocol procedures with particular attention to inclusion/exclusion criteria, enrollment goals, adverse events, primary efficacy variables and GCP compliance) | ||
| • | Informed consent procedure | ||
| • | Randomization procedure | ||
| • | AE/SAE reporting | ||
| • | CRF completion and error correction/need for adequate source documentation | ||
| • | Maintenance of the investigator binder and site visit log | ||
| • | Laboratory sample/photography sample handling procedures and results reporting procedures | ||
| • | Clinical supply dispensation, accountability and storage procedures |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 8
| • | Source document verification | ||
| • | CRF completion | ||
| • | Expedient data retrieval and query resolution | ||
| • | Drug accountability | ||
| • | Check and review of the regulatory binder and its contents | ||
| • | Clinical supply inventory | ||
| • | SAE reporting | ||
| • | Enrollment issues and targets | ||
| • | Protocol amendments | ||
| • | Significant protocol deviations | ||
| • | Acceptability of facilities | ||
| • | Personnel changes | ||
| • | Updated regulatory documentation | ||
| • | Laboratory sample/photography sample handling |
| • | Reason for termination (if study is not complete) | ||
| • | Reconciliation and removal of clinical supplies per The Sponsor’s requirements (including hazardous materials) | ||
| • | All original CRFs retrieved from the site at the previous visit | ||
| • | Signed informed consents retained by investigator | ||
| • | Record retention requirements | ||
| • | Notification to IRB or ethics committee of study termination | ||
| • | Collection of randomization codes for return to The Sponsor | ||
| • | Resolution of all data queries | ||
| • | Investigator binder contents (complete and updated) | ||
| • | Informed consent log | ||
| • | Financial agreements and disclosure (filed separately) | ||
| • | Signature log and screening log | ||
| • | Site visit and subject enrollment log | ||
| • | Laboratory certification and renewals | ||
| • | Summary/discussion of study from investigator and/or staff |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 9
| • | Discussion of study protocol and any amendments to the protocol | ||
| • | Inclusion/exclusion questions or issues | ||
| • | Review of clinical laboratory results | ||
| • | New staff or team member orientation | ||
| • | Additional site training | ||
| • | Clinical supply activities or issues | ||
| • | Enrollment updates (via weekly fax updates) | ||
| • | AE and SAE updates | ||
| • | Data query resolution | ||
| • | Scheduling activities |
| • | Processing financials records for all of the patients in the study | ||
| • | Issue initial and interim payments for each investigator | ||
| • | Reconciling all payments to each of the investigators prior to final payments | ||
| • | Tracking account administration with Omnicare CR’s finance group | ||
| • | Maintaining IRS W-9 forms and all relevant and government reports | ||
| • | Reporting excess grants from sites at study end |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 10
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 11
| • | Addressing medical inquiries, internal or external | ||
| • | Reviewing of clinical documentation (protocol, draft CRF, sample informed consent form) | ||
| • | Project-specific medical training | ||
| • | Evaluating patient eligibility (in conjunction with the Medical Director of the Sponsor) | ||
| • | Participating in team meetings | ||
| • | Review of Safety Data, such as laboratory results, ECGs, SAEs, AE Listings | ||
| • | Reviewing study reports, regulatory submissions and study manuscripts |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 12
| • | Overseeing and supporting the Lead CDA throughout the completion of setup and completion of all data management activities | ||
| • | Review and approval of the Data Management Plan. The Data Manager will assure the receipt of the Sponsor’s approval prior to the initiation of any tasks outlined in this plan | ||
| • | Supporting the development of the CRF completion guidelines |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 13
| • | Maintain ongoing communication with Sponsor team members and Omnicare CR’s Project Team Members | ||
| • | Development of resource and contingency plans to ensure appropriate project staffing throughout the study, based on the Data Retrieval Plan provided by the CTM. |
| • | Prioritize of Clinical Data Management tasks | ||
| • | Direct the daily Clinical Data Management team task assignments | ||
| • | Monitor the status of task and work load | ||
| • | Oversee Project training for Clinical Data Management team members. | ||
| • | Liaise with Clinical Trial Managers to ensure expectations for recording data accurately are communicated to the Project Team and the study site | ||
| • | Manage the query generation and final resolution | ||
| • | Proactively address data quality issues to reduce query generation | ||
| • | Liaise with the Clinical Trial Managers for timely query resolution | ||
| • | Ensure high quality and timely data management deliverables | ||
| • | Provide feedback to the Clinical Monitoring staff on query trends | ||
| • | Provide backup support to the Clinical Data Manager | ||
| • | Ensure a cohesive team that maintains high quality and data consistency | ||
| • | Provide status updates to both the Omnicare CR internal Project Team and the Sponsor team members |
| • | Project data flow | ||
| • | Database development overview | ||
| • | Edit specifications | ||
| • | Data entry guidelines | ||
| • | Data handling guidelines | ||
| • | Study assumptions (Level 1) | ||
| • | SAE reconciliation process | ||
| • | External data load procedures / External Data Cleaning parameters / Discrepancy identification flow | ||
| • | Dictionary coding guidelines and processes | ||
| • | Database closure procedures |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 14
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 15
| • | Reviewing the CRF data for obvious corrections and potential queries via the electronic Edit checks and the Manual review checks listed in the Data Management Plan | ||
| • | Applying any agreed upon self evident corrections or study assumptions | ||
| • | Transmitting queries to investigative sites with a copy forwarded to Project Management | ||
| • | Logging queries (issued and resolved) into Oracle Clinical Discrepancy Management System | ||
| • | Working with the Clinical Trials Manager to ensure all Data Clarification Forms (DCFs) are signed by the investigator and received in house via fax or mail. |
| • | Quality data | ||
| • | On-time delivery of Final Clean Clinical Study Databases | ||
| • | No surprises when preparing for data analysis |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 16
| • | Design and maintain the clinical database with Omnicare CR’s current version (at time of database build) of MedDRA for coding Adverse Events and Medical History terms, and WHO-Drug for coding Medications | ||
| • | Utilize standard coding conventions as listed in the Data Management Plan for the mapping procedures. If Sponsor requires specific coding guidelines they must be provided prior to the start of the coding work and will be included in the Data Management Plan | ||
| • | Utilize an automated process using Oracle TMS to map literal text to the corresponding term in the dictionary | ||
| • | Research, code and review unmapped terms with a dedicated team |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 17
| • | Cumulative CRF / DCF Status | ||
| • | Cumulative CRFs by Site | ||
| • | Outstanding DCFs by Site | ||
| • | Resolved DCFs by Site | ||
| • | Weekly Metrics: Data Processing |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 18
| • | Initialize an internal Quality Control project tracking system which tracks the status of the program, the validation of the program output, the owners of the files and the dates that the events were completed | ||
| • | Customize project specific macros and header files for program development | ||
| • | Create a cross reference file for data displays to SAS program files | ||
| • | Review of client programming style guidelines if applicable and incorporate into programming specifications if applicable | ||
| • | Hold at least one internal kick-off meeting for the Biometrics team members |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 19
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 20
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 21
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 22
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 23
| • | Investigator Recruitment / Essential Document Tracking | ||
| • | Patient Enrollment (patient accrual) by site and by trial projected and actual | ||
| • | Monitoring Statue and Monitor Visit Scheduling | ||
| • | CRF pages ready for review at site (projected/actual), CRF pages collected as related to patient and monitoring visit schedules | ||
| • | Trip Reporting — electronic trip reports |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 24
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 25
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 26
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 27
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 28
| Omnicare CR, Inc. Clinical Budget for: | ||
Sponsor:
|
Xxxxxx | |
Compound:
|
PEP005 Topical Gel | |
Study:
|
A multi-center, randomized, double-blind, double-dummy, vehicle-controlled sequential cohort study to determine the safety of PEP005 0.025% and 0.05% topical gel in patients with actinic keratoses | |
PCN:
|
KO1605 | |
| Unit | Estimated | Estimated | |||||||||||||||||||||||||||
| Services | Unit | # Units | Cost | Fees | Pass-Thru | Total Cost | |||||||||||||||||||||||
| A. Study Management | |||||||||||||||||||||||||||||
| • | Project Director —
Americas (assumes
****% FTE) |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Project
Administrative
Support/Coordination
- Americas
(includes support
for all functional
areas) (assumes ****%
FTE) |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Draft Informed
Consent Review |
Review | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Sub-Total Study Management |
**** | **** | **** | ||||||||||||||||||||||||||
| B. Clinical Trial Initiation | |||||||||||||||||||||||||||||
| • | Site Screening and Identification (assumes Xxxxxx will perform this task) | ||||||||||||||||||||||||||||
> Zone 1 |
Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Site Recruitment (assumes Xxxxxx will perform this task) | ||||||||||||||||||||||||||||
> Zone 1 |
Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • |
Study Master File |
||||||||||||||||||||||||||||
> Americas: ****
sites for ****
months |
Site Months | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Regulatory Document Collection | ||||||||||||||||||||||||||||
> Zone 1 |
Site | **** | **** | **** | **** | ||||||||||||||||||||||||
| Central IRB (pass-throughs are estimates only; actual fees will be billed based on specific IRB fees) | Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
Local IRB
(pass-throughs are
estimates only;
actual fees will be
billed based on
specific IRB fees) |
Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Protocol Amendments — excluding ICF change | ||||||||||||||||||||||||||||
> Xxxx 0 |
Xxxxxxxxxx/xxxx | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Protocol Amendments — including ICF change | ||||||||||||||||||||||||||||
|
> Xxxx 0 |
Xxxxxxxxxx/xxxx | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 29 |
| Unit | Estimated | Estimated | |||||||||||||||||||||||||||
| Services | Unit | # Units | Cost | Fees | Pass-Thru | Total Cost | |||||||||||||||||||||||
| • | Investigator Agreement Negotiation — Standard (actuals will be billed) | ||||||||||||||||||||||||||||
| > Americas | Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Investigator Agreement Negotiation — Complex (actuals will be billed) | ||||||||||||||||||||||||||||
| > Americas | Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Investigator Agreement Amendments — Standard | ||||||||||||||||||||||||||||
| > Americas | Amendments/site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • |
Letters of Indemnification (US
sites only) |
Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • |
Facility Letters |
||||||||||||||||||||||||||||
># Standard Facility Letters |
Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
|
># Complex Facility Letters |
Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • |
Notice Letters |
Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • |
IND Safety Report |
||||||||||||||||||||||||||||
| >Zone 1 | Reports/Site | **** | **** | **** | **** | ||||||||||||||||||||||||
| • |
Investigator Brochure Updates |
||||||||||||||||||||||||||||
| >Zone 1 | IB Updates/Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Collect Financial Disclosure at Site Closeout | ||||||||||||||||||||||||||||
| >Zone 1 | Site | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • |
Vendor/Lab Service Agreements
— Americas |
Project | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigators’ Meeting Coordination (pass-through is for investigator travel only-other pass-throughs are indicated below) | ||||||||||||||||||||||||||||
|
> Coordination, Meeting 0
Xxxxx Xxxxxxx |
Meeting | **** | **** | **** | **** | **** | |||||||||||||||||||||||
|
> Per Attendee, Meeting 1
North America |
Attendee | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • |
Investigators Meeting Preparation |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigators’ Meeting Attendance (OCR attendees x days) | ||||||||||||||||||||||||||||
|
Project Director — Americas |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||||
|
CTM — Americas |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||||
|
Medical Monitor — Americas |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||||
|
Project Coordinator |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||||
|
CRA Attendees |
|||||||||||||||||||||||||||||
|
>Zone 1: **** CRAs |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||||
|
Client Attendee Travel |
Attendee | **** | **** | **** | **** | **** | |||||||||||||||||||||||
|
Meeting Coordinators |
Attendee | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| Sub-Total Clinical Trial Initiation | **** | **** | **** | ||||||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 30 |
| C. Clinical Trial Management | ||||||||||||||||||||||||||
| • | Clinical Trial Manager - Americas (assumes ****% FTE) |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||
| • | Clinical Monitoring |
|||||||||||||||||||||||||
| >Site Qualification Visit | ||||||||||||||||||||||||||
>Zone 1: **** hrs
on-site, **** hrs for prep,
follow-up, and reports,
and **** hrs for travel
|
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||
>Site Initiation Visit |
||||||||||||||||||||||||||
>Zone 1: **** hrs
on-site, **** hrs for prep,
follow-up, and reports,
and **** hrs for travel
|
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||
>Site Interims Visit |
||||||||||||||||||||||||||
>Zone 1: assume ****
visits/site, **** hrs on-
site, **** hrs for prep,
follow-up, and reports,
and **** hrs for travel
|
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||
| >Additional Day Interim Visits (based on **** hour day; visits are total per zone; actuals will be billed) | ||||||||||||||||||||||||||
>Zone 1: assume ****
days for Zone pool of
visits
|
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||
| >Site Close-out Visit | ||||||||||||||||||||||||||
>Zone 1: **** hrs on-
site, **** hrs for prep,
follow-up, and reports,
and **** hrs for travel
|
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||
| • | Site Maintenance for **** hrs/month/site (**** sites x **** enrollment and treatment months) | |||||||||||||||||||||||||
>Zone 1: **** sites
|
Site Months | **** | **** | **** | **** | **** | ||||||||||||||||||||
| • | CRA Monthly Teleconferences |
|||||||||||||||||||||||||
>Zone 1: **** CRAS
|
Months | **** | **** | **** | **** | **** | ||||||||||||||||||||
| • | Project Team Training (To be held in conjunction with Investigator Meeting) | |||||||||||||||||||||||||
Project Director - Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
CTM — Americas
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Medical Monitor - Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Project Coordinator
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
CRA Attendees
|
Days | |||||||||||||||||||||||||
>Zone 1 CRAs: **** CRAs
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
| • | Sponsor Meeting Attendance (assumes **** face-to-face meetings and **** one-hour teleconferences, not all positions participate in all meetings; xxxx on actuals) | |||||||||||||||||||||||||
Project Director - Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
CTM — Americas
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Data Manager — Int’l
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Statistician — Int’l
|
**** | **** | **** | **** | **** | |||||||||||||||||||||
Sr. Writer — Americas
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 31
Project Coordinator
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Clinical Data Analyst
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Programmer
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
| • | Project Team Teleconference Attendance (assumes **** meetings; not all positions participate in all meetings; xxxx on actuals) | |||||||||||||||||||||||||
Project Director - Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
CTM — Americas
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Data
Manager — Int’l
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Medical Monitor - Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Safety Officer - Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Statistician — Int’l
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Sr. Writer — Americas
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
CQA Manager- Americas
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Study Initiation Manager |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Project Coordinator
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Clinical Data Analyst
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Contract Negotiator
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
Programmer
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||
| • | Clinical Grants Administration | |||||||||||||||||||||||||
| >Grant Payment Set-up | ||||||||||||||||||||||||||
>Zone 1
|
Sites | **** | **** | **** | **** | |||||||||||||||||||||
| > Grant
Management (takes place for active sites from site set-up through
**** days after site close-out; estimated amounts; actuals will be billed based on number of active sites set up) |
||||||||||||||||||||||||||
>Zone 1
|
Site Quarters | **** | **** | **** | **** | |||||||||||||||||||||
| >Payment
Processing (estimated based on **** payments/site; actuals, including
investigator and site related payment and any miscellaneous payments, actuals will be billed; pass through costs are related to photocopying and postage) |
||||||||||||||||||||||||||
>Zone 1: **** sites
|
Payments | **** | **** | **** | **** | **** | ||||||||||||||||||||
>Estimated
Investigator Grants
(Includes
Administrative,
Study Coordinator,
and Investigator
fees; excludes tests
in the synopsis that
will be performed by
the central
laboratory; estimate
based upon draft
synopsis provided
and may change upon
review of the final
protocol)
|
Patient | **** | **** | **** | **** | **** | ||||||||||||||||||||
| Screen Fail Patient - Americas |
**** | **** | **** | **** | ||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 32
| Enrolled Patient — Americas | **** | **** | **** | **** | ||||||||||||||||||||||||
| • | Refund Checks (if
needed; actuals
will be billed) |
Refund | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Central Laboratory
Services (**** passthroughs) |
Project | **** | **** | **** | **** | ||||||||||||||||||||||
| • | **** (based
upon **** Sites) |
Project | **** | **** | **** | |||||||||||||||||||||||
| Sub Total Clinical Trial Management | **** | **** | **** | |||||||||||||||||||||||||
| D. Safety and Medical | ||||||||||||||||||||||||||||
| • | Medical Monitoring availability during business hours (flat fee of **** hours per month from first patient in through last patient out) | |||||||||||||||||||||||||||
| >Americas | Months | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| Greater than **** hours per month will be billed at hourly rate (from first patient in through last patient out); Billed on actuals | ||||||||||||||||||||||||||||
| >Americas | Hours | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Medical Monitoring during Study Start Up and Study Close-out (Billed on actuals) | |||||||||||||||||||||||||||
| >Americas | Hours | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Safety Coordinator |
|||||||||||||||||||||||||||
| >Americas — assumes **** day per month for **** months | Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | SAE Reporting (with
initial descriptive
summary) Billed on
actuals |
Report | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | SAE Reporting
(Follow-up/Revision
Reports >****)
Billed on actuals |
Report | **** | **** | **** | **** | **** | |||||||||||||||||||||
| > Data Entry into **** (actuals will be billed) | SAE | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Medical Review of
Protocol (xxxx on
actuals) |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||
| Sub-Total Safety and Medical | **** | **** | **** | |||||||||||||||||||||||||
| E. Clinical Data Management | ||||||||||||||||||||||||||||
| • | Clinical Data
Management
Oversight — Int’l
(CDM Manager
assumes ****% FTE x
**** months for
Start-up, ****% FTE
for **** months for
Duration, and ****%
FTE for **** months
for Close-out) |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 33
| • | Lead CDA — Int’l
(assumes ****% FTE x
**** months, ****% FTE x
**** months, and ****%
FTE for **** months) |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Development of Data
Management Plan
(includes **** major
and **** minor
revision; add’l
revisions will be
billed at per diem
rates) |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | CRF Design (assumes
**** page CRF, ****
unique CRF pages);
includes **** major
and **** minor
revision |
Project | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Database
Development,
Testing and
Maintenance
(assumes **** page
CRF, **** unique CRF
pages) |
Project | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Estimated Data Entry (actuals will be billed) | ||||||||||||||||||||||||||||
| > Pages in Int’l | Pages | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Estimated Data Review and Query Resolution (assumes **** issue per **** CRF pages, **** manual checks and the application of **** study assumption for **** CRF pages); assumes **** CRF pages per enrolled patient, **** pages per screen failure patient and **** pages per drop out patient. Pages will be billed on actuals. | ||||||||||||||||||||||||||||
| > CRF Pages | Page | **** | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Quality control
check — critical
items |
Patient | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Quality control
check — full case |
Patient | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Edit Development
(actuals will be
billed) |
Edits | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | CRF Tracking
(includes all
ancillary pages;
actuals will be
billed) |
Page | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Dictionary Med
remapping — ATC
Meds (actuals will
be billed) |
Term | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Dictionary Coding
of Adverse Event
terms to
MedDRA(estimated to
be **** per patient;
actuals will be
billed) |
Term | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 34
| • | Dictionary Coding of |
Term | **** | **** | **** | **** | **** | |||||||||||||||||
Medication Terms
(estimated to be **** per
patient; actuals will
be billed) |
||||||||||||||||||||||||
| • | External Vendor — Initial Load (actuals will be billed) | |||||||||||||||||||||||
Initial Load |
Load | **** | **** | **** | **** | **** | ||||||||||||||||||
>Subsequent Load |
Loads | **** | **** | **** | **** | **** | ||||||||||||||||||
(actuals will be
billed) |
||||||||||||||||||||||||
Lab Visit Verification |
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||
(**** visits x ****
patients; actuals will
be billed) |
||||||||||||||||||||||||
| • | Reconciliation of the |
SAE | **** | **** | **** | **** | ||||||||||||||||||
Safety and Clinical
Database (actuals will
be billed) |
||||||||||||||||||||||||
| • | Interim Database Close (assumes clean database; based on # patients in close — actuals will be billed) | |||||||||||||||||||||||
| • | >1st
Interim Close |
Patients | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Protocol Deviation Log |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
Load |
||||||||||||||||||||||||
| Sub-Total Clinical Data Management | **** | **** | **** | |||||||||||||||||||||
| F. Biometrics Analysis | ||||||||||||||||||||||||
| • | Biometrics Team Manager |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Biometrics CRF Review |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Statistical Input into |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
Protocol |
||||||||||||||||||||||||
| • | Project Data Setup |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Statistical Plan |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Sample Size/Power |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
Determination |
||||||||||||||||||||||||
| • | Randomization Schedule |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Design of Table Shells |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
(Mocks) |
||||||||||||||||||||||||
| • | Programming/QC of Data Displays (actuals will be billed). | |||||||||||||||||||||||
| Unique Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||
| Repeat Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||
| Unique Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||
| Repeat Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||
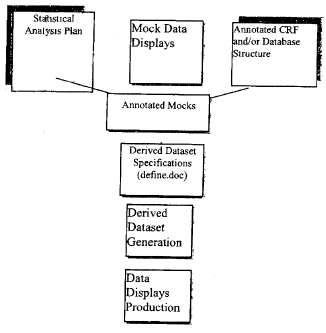
| Unique Figures | Figure/Graph | **** | **** | **** | **** | **** | ||||||||||||||||||
| Repeat Figures | Figure/Graph | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Custom Derived Data Sets |
|||||||||||||||||||||||
>Initial
(Assumes **** |
Project | **** | **** | **** | **** | **** | ||||||||||||||||||
datasets) |
||||||||||||||||||||||||
>Subsequent |
Dataset | **** | **** | **** | **** | **** | ||||||||||||||||||
(Assumes ****
datasets) |
||||||||||||||||||||||||
| • | Programmatic Evaluability/Outcome |
|||||||||||||||||||||||
>Regular or Advanced |
Project | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Statistical Analysis |
|||||||||||||||||||||||
>Regular |
Project | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | FDA Item 11 |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 35
| • | Standard Data Transfer
|
Transfer | **** | **** | **** | **** | **** | |||||||||||||||||
>Initial |
Transfer | **** | **** | **** | **** | **** | ||||||||||||||||||
>Subsequent |
Transfer | **** | **** | **** | **** | **** | ||||||||||||||||||
| Add’l Stats consulting, meeting attendance, etc., will be charged at per diem rates as follows: | ||||||||||||||||||||||||
Team
Leader |
Days | **** | ||||||||||||||||||||||
Statistician |
Days | **** | ||||||||||||||||||||||
Programmer |
Days | **** | ||||||||||||||||||||||
| • | DSMB Contracts |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
(assumes Xxxxxx
will recruit
participants) |
||||||||||||||||||||||||
| • | DSMB Orientation Meeting (organize, attend, and follow up) | |||||||||||||||||||||||
Project Director |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||
Project Coordinator |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | DSMB Meeting |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
(Project Director
Attendance,
Preparation, and
Follow Up) |
||||||||||||||||||||||||
| • | DSMB Meeting |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
(Project
Coordinator
Support) |
||||||||||||||||||||||||
| • | DSMB Meeting |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
(Statistician
Attendance and
Preparation) |
||||||||||||||||||||||||
| • | Safety DSMB Data |
Unique Tables | **** | **** | **** | **** | **** | |||||||||||||||||
Displays |
||||||||||||||||||||||||
| Repeat Tables | **** | **** | **** | **** | **** | |||||||||||||||||||
| Unique Listings | **** | **** | **** | **** | **** | |||||||||||||||||||
| Derived Datasets | **** | **** | **** | **** | **** | |||||||||||||||||||
| • | Write DSMB Charter |
|||||||||||||||||||||||
Clinical Writer |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||
Statistician |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Statistical |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
Appendix document
to chapter |
||||||||||||||||||||||||
| • | DSMB Contractor |
Project | **** | **** | **** | |||||||||||||||||||
Sub-Total Biometrics |
**** | **** | **** | |||||||||||||||||||||
| G. Clinical Writing | All assumes using OCR SOPs. If using Sponsor SOPs/templates, all CW is custom priced | |||||||||||||||||||||||
| • | Clinical Study Report: |
|||||||||||||||||||||||
Phase II/III Report |
Report | **** | **** | **** | **** | **** | ||||||||||||||||||
> includes ****
major (up to ****
days) and
**** minor (up to ****
day) revision
> assumes up to
**** tables and ****
listing provided
for
summarization>
assumes OCR SOPs
and CSR template
> fee does not
include CSR
appendices
(including TLs) |
||||||||||||||||||||||||
> Tables |
Report | **** | **** | **** | **** | |||||||||||||||||||
(greater than ****) |
||||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 36
| • | Narratives (actuals |
Narrative | **** | **** | **** | **** | **** | |||||||||||||||||
will be billed) |
||||||||||||||||||||||||
| • | Clinical writing |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
Input to SAP |
||||||||||||||||||||||||
| Attendance at Sponsor Requested Meetings: | ||||||||||||||||||||||||
| Attendance at Sponsor requested meetings (teleconferences/video conferences or client review/planning meetings at Sponsor/Omnicare CR, Inc.) will be billed to Sponsor according to the following per diem rates: | ||||||||||||||||||||||||
Director: |
$ | **** | ||||||||||||||||||||||
Senior Writer: |
$ | **** | ||||||||||||||||||||||
Clinical Writer: |
$ | **** | ||||||||||||||||||||||
| Sponsor will be billed for actual time expended. | ||||||||||||||||||||||||
Sub-Total Clinical Writing |
**** | **** | **** | |||||||||||||||||||||
| H. Technology | ||||||||||||||||||||||||
| • | OmnieView |
|||||||||||||||||||||||
>Monthly Maintenance |
Months | **** | **** | **** | **** | **** | ||||||||||||||||||
Sub-Total Technology |
**** | **** | **** | |||||||||||||||||||||
| I. Regulatory Services | ||||||||||||||||||||||||
| • | CRF Filing and Reconciliation (xxxx |
Pages | **** | **** | **** | **** | **** | |||||||||||||||||
on actuals) |
||||||||||||||||||||||||
| • | Return of CRF (hard copy); xxxx on |
Pages | **** | **** | **** | **** | **** | |||||||||||||||||
actuals |
||||||||||||||||||||||||
| • | Return of Investigator and Study-Wide |
Sites | **** | **** | **** | **** | **** | |||||||||||||||||
Documents (paper) |
||||||||||||||||||||||||
| • | Regulatory Site Drug Release Approval |
# Sites | **** | **** | **** | **** | ||||||||||||||||||
| • | Compilation of Clinical Study Report Appendices (electronic copy) | |||||||||||||||||||||||
>Setup
and Management Fee |
Report | **** | **** | **** | **** | **** | ||||||||||||||||||
>Scanning (without cleaning); |
Pages | **** | **** | **** | **** | **** | ||||||||||||||||||
actuals
will be billed |
||||||||||||||||||||||||
>Scanning and cleaning; actuals |
Pages | **** | **** | **** | **** | **** | ||||||||||||||||||
will be
billed |
||||||||||||||||||||||||
>Volumes (fee does not include |
Volume | **** | **** | **** | **** | **** | ||||||||||||||||||
hyper-linking); actuals will be billed |
||||||||||||||||||||||||
| • | Regulatory Consulting |
Days | **** | **** | **** | **** | ||||||||||||||||||
(Vice President Americas) |
||||||||||||||||||||||||
Sub-Total Regulatory Services |
**** | **** | **** | |||||||||||||||||||||
| J. Clinical Quality Assurance | ||||||||||||||||||||||||
| • | Quality Plan |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
| • | CQA Site Audits — includes preparation and travel time, audit time, audit follow-up, and Audit Report and Audit Certificate generation | |||||||||||||||||||||||
>Assumes ****
sites in Americas |
Sites | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Pre-Regulatory |
Audit | **** | **** | **** | **** | **** | |||||||||||||||||
Inspection
Audits |
||||||||||||||||||||||||
Sub-Total Clinical Quality Assurance |
**** | **** | **** | |||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 37
| K. Clinical Supplies Management | ||||||||||||||||||||||||
| • | Receipt and Inventory of Study
Drug (Americas) |
Per 100 patients | **** | **** | **** | **** | ||||||||||||||||||
| • | Receipt and Inventory of Study
Drug (Americas) |
Batch | **** | **** | **** | **** | ||||||||||||||||||
| • | Storage of
study drug (Americas)
storage conditions: refrigerated (2°C-8°C) |
Month | **** | **** | **** | **** | ||||||||||||||||||
| • | Shipment Preparation and
shipping of Study
Drug to sites in
Americas. Shipping
conditions: refrigerated (2°C-8°C), **** sites, **** shipments per site (Pass through includes insulated shipping container and temperature recorder. Xxxxxx must supply a qualified shipping container or a qualification can be done at an extra fee.) |
Shipment | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Manage
General Study drug Issues
(Americas) **** days
per month |
Month | **** | **** | **** | **** | ||||||||||||||||||
| • | Project
meetings with Pharmacist’s
attendance |
Day | **** | **** | **** | **** | ||||||||||||||||||
| • | Receipt of
Returned Drug Americas (****
receipt per site,
**** sites) |
Receipt | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Storage of
returned drug at room
temperature
(Americas) |
Month | **** | **** | **** | **** | ||||||||||||||||||
| • | Return of
study drug to Sponsor or
Certified
Destructor (Incl.
provision of
Certificate of
destruction) |
Destruction run | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Final reconciliation
(Americas) |
Day | **** | **** | **** | **** | ||||||||||||||||||
Sub-Total
Clinical Supplies Management |
**** | **** | **** | |||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 38
| L. Miscellaneous Pass-Through Expenses | ||||||||||||||||||||||||
| • | CRF Printing (xxxx on actuals) | Pages | **** | **** | **** | **** | ||||||||||||||||||
| • | Investigator Binders (includes printing, copying and shipping; xxxx on actuals) | Binder | **** | **** | **** | **** | ||||||||||||||||||
| • | Shipment of Start-up Packet (xxxx on actuals) | Sites | **** | **** | **** | |||||||||||||||||||
| • | Courier Cost for Drug Shipments to Sites (xxxx on actuals) | Shipment | **** | **** | **** | **** | ||||||||||||||||||
| • | Ship CRFs (xxxx on actuals) | Box | **** | **** | **** | **** | ||||||||||||||||||
| • | Ship CRF from OCR back to Sites (xxxx on actuals) | Box | **** | **** | **** | **** | ||||||||||||||||||
| • | Copying (xxxx on actuals) | Pages | **** | **** | **** | **** | ||||||||||||||||||
| • | Investigator Brochure Printing (xxxx on actuals) | Pages | **** | **** | **** | **** | ||||||||||||||||||
| • | Protocol Printing (xxxx on actuals) |
Pages | **** | **** | **** | **** | ||||||||||||||||||
| • | Informed
Consent Printing (xxxx on actuals) |
Pages | **** | **** | **** | **** | ||||||||||||||||||
| Sub-Total Miscellaneous Pass Through Expenses | **** | **** | ||||||||||||||||||||||
| Estimated Services Budget | **** | |||||||||||||||||||||||
| Estimated Pass Through | **** | |||||||||||||||||||||||
| Total Estimated Budget | **** | |||||||||||||||||||||||
| Optional Services | ||||||||||||||||||||||||
| Total Optional Services | ||||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 39
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 40
| • | Travel | |
| • | Delivery costs | |
| • | CRF and other printing or copying costs | |
| • | Investigator Meeting costs | |
| • | Telecommunication costs (which may include telephone, fax, pager, conference calls, or PC connectivity charges) |
Wachovia Bank
ABA # ****
Acct # ****
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 41
| BY AND BETWEEN: | ||||||||||||||
| Xxxxxx Operations Pty Ltd. | Omnicare CR, Inc. | |||||||||||||
| By: | /s/ Xxxxx Xxxxxxx | By: | /s/ Xxxx Xxxxx | |||||||||||
| Name: | Xxxxx Xxxxxxx Chief Scientific Officer and Vice President, | Name: Title: |
Xxxx Xxxxx CEO |
|||||||||||
| Title: | Research & Development | |||||||||||||
| Date: 17 October 2006 | Date: 10/24/06 | |||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 42
between Xxxxxx Operations Pty Ltd. and Omnicare CR, Inc.,
dated 1st June 2005.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 1 | Xxxxxx Operations Pty Ltd |
| Estimated Service | ||||
| Reference | Changes and/or Additions | Fee (US$) | ||
Pursuant to Exhibit
I, Omnicare CR will
provide additional statistical
services for
Sponsor’s
requirements
|
Additional data displays presented within the Data Monitoring Committee Statistical Analysis Plan: |
|||
•
Data Tables (original contract
included 10 unique and 5 repeat; actual
displays were 17 unique and 8 repeat tables): |
||||
-
7 unique tables @ US$****/table
|
US$**** | |||
-
3 repeat tables @ US$****/table
|
US$**** | |||
• Data Listings (original contract
included 2 unique and 0 repeat; actual
displays were 12 unique and 4 repeat
listings): |
||||
-
10 unique listings @ US$****/table
|
US$**** | |||
-
4 repeat listings @ US$****/table
|
US$**** | |||
| Total Estimated Service Fees | US$***** | |||
| Total Estimated Pass Through Expenses | ****** | |||
| Total Estimated Budget | US$**** | |||
| * | The Estimated Service Fees set forth above represent the original unit costs set forth in the Agreement and are subject to any annual price increase(s) applied against the original unit costs. | |
| ** | Sponsor will be billed for actual Pass-Through Expenses incurred in support of the Project. |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 2 | Xxxxxx Operations Pty Ltd |
Bank Name: Wachovia Bank
ABA Number: ****
Account Number: ****
| ACCEPTANCE | ||||||||||||
| BY AND BETWEEN: | ||||||||||||
| Xxxxxx Operations Pty Ltd. | Omnicare CR, Inc. | |||||||||||
| By: | /s/ Xxxxxxx Xxxxxxxx | By: | /s/ Xxxx Xxxxx | |||||||||
| Name: | Xxxxxxx Xxxxxxxx | Name: | Xxxx Xxxxx | |||||||||
| Title: | Director Clinical Dev. | Title: | CEO | |||||||||
| Dated: 27 November 06 | Dated: 12/06/06 | |||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 3 | Xxxxxx Operations Pty Ltd |
Initial Sponsor Notification Form
|
 |
Protocol Number: PEP005-006
|
Date of Request; 16NOV2006 | Omnicare PCN: KO1605.01 | ||
Omnicare Project Director:
|
**** | |||
Omnicare Account Director:
|
**** | |||
Sponsor Study Manager:
|
Xxxxxxx Xxxxxxxx | |||
Sponsor Outsourcing Manager: |
||||
Individual and Department making request:
|
****, Biometrics | |||
| Brief Description of Project Scope Change: | ||||
| The number of data displays presented within the Data Monitoring Committee Statistical Analysis Plan and reviewed by the DMC exceeds that presented within Exhibit I. | ||||
| Exhibit I has 10 unique/5 repeat tables and 2 unique/0 repeat listings for the DSMB SAP. The current DMC SAP has 17 unique/8 repeat tables and 12 unique/4 repeat listings. This represents an increase of 7 unique tables, 3 repeat tables, 10 unique listings and 4 repeat listings with an estimated additional cost of $**** USD. | ||||
Implementation of this request will
affect the budget as follows (provide
estimate of anticipated costs associated
with change, if available):
|
þ Increase o Decrease |
|||
| o Other (specify) | ||||
/s/
Xxxxxxx Xxxxxxxx
|
17/11/06 | |||
Sponsor Signature
|
Date |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Omnicare Clinical Research CONFIDENTIAL |
Page 1 of 1 |
between Xxxxxx Operations Pty Ltd and Omnicare CR, Inc.,
dated June 1, 2005
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 40
| GENERAL STUDY INFORMATION | ||||||||
Compound
|
PEP005 Topical Gel | |||||||
Therapeutic Area / Indication
|
Superficial basal cell carcinoma | |||||||
Study Phase
|
IIa | |||||||
Bid Currency
|
USD $ | |||||||
| STUDY SITES | ||||||||
Number of Investigative Sites
|
6 | |||||||
Number of Back-up Investigative Sites
|
— | |||||||
Number of Sites per Country |
||||||||
• US
|
6 | |||||||
| PATIENTS | ||||||||
Number of Screened Patients
|
116 (58 per treatment arm) | |||||||
Number of Enrolled Patients
|
58 per treatment arm (2 treatment arms) Budget based on total 116 | |||||||
Number of Completed Patients
|
116 (58 per treatment arm) (assumed maximum # of patients for budget purposes) | |||||||
| PROJECT MANAGEMENT | ||||||||
Project Director — Americas
|
****% FTE for **** months; **** Days | |||||||
Clinical Trial Manager — Americas
|
****% FTE for **** months; **** Days | |||||||
Project Coordinator — Americas
|
****% FTE for **** months; **** Days | |||||||
| STUDY START-UP ACTIVITIES | ||||||||
Number of Investigator Sites
Screened / Recruited
|
6 sites, pre-selected by Sponsor | |||||||
Submission to Ethics Committees / IRB
|
1 Central IRB | |||||||
Submission to Regulatory Authority
|
Sponsor | |||||||
IMPD Preparation (Type)
|
No Omnicare involvement | |||||||
| MONITORING AND SITE MANAGEMENT | ||||||||
Number of CRAs
|
2 | |||||||
| Not required | ||||||||
Number of Initiation Visits
|
**** visits; assumes **** hours on-site; **** hours travel; **** preparation, report and follow-up | |||||||
Frequency of interim Visits
|
Assumes every **** weeks | |||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 2
| MONITORING AND SITE MANAGEMENT | ||||||||
Number of Interim Visits
|
**** visits; assumes **** hours on-site; **** hours travel; **** preparation, report and follow-up | |||||||
Pool of Additional One-Day Visits
|
TBD if required | |||||||
Number of Close Out Visits
|
**** visits; assumes **** hours on-site, **** hours travel; **** preparation, report and follow-up | |||||||
Site Maintenance
|
**** hours per site, for **** sites for **** months | |||||||
| SPONSOR MEETINGS | ||||||||
Number and Location of Investigator Meeting
|
One in the U.S. | |||||||
Omnicare Investigator Meeting Attendees
|
See budget | |||||||
Project Team Training
|
One in the U.S, in combination with investigators’ Meeting | |||||||
Kick-off Meeting
|
NA | |||||||
Number of Team Teleconferences and Attendees
|
Monthly CRA TCs | |||||||
| DATA MANAGEMENT | ||||||||
Lead Data Manager — International
|
****% FTE for **** months; **** Days | |||||||
Lead CDA — International
|
****% FTE for **** months; **** Days | |||||||
Number of CRF Pages — Enrolled Patient
|
50 pages per patient (25 Unique/25 Repeat) | |||||||
Number of Adverse Event Terms per Patient
|
3 | |||||||
Number of Con Med Terms per Patient
|
7 | |||||||
Number of Medical Histories per Patient
|
n/a | |||||||
Number of Edits
|
200 | |||||||
Number/Type of Laboratory
|
1 Central Lab 12 data imports (monthly) | |||||||
Number of Queries per Patient
|
**** per **** CRF pages | |||||||
| BIOMETRICS | ||||||||
Biometrics Team Lead — International
|
**** Days per Month **** Days in total | |||||||
Number of Tables
|
50 Unique/35 Repeat | |||||||
Number of Listings
|
22 Unique / 5 Repeat | |||||||
Number of Figures
|
0 Unique / 0 Repeat | |||||||
Number of Interim Analysis
|
n/a | |||||||
Number of Transfers
|
0 | |||||||
| SAFETY/MEDICAL | ||||||||
Medical Monitor — Americas
|
**** Days per Month | |||||||
Number of SAEs (with narratives)
|
4 | |||||||
Number of Days Safety Coordinator — Americas
|
**** Days | |||||||
Number of Months **** Maintenance
|
12 | |||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 3
| MEDICAL WRITING | |||||
Study Protocol Development
|
Provided by Sponsor | ||||
Clinical Study Report
|
Assuming using Omnicare SOPs, includes **** major (**** days) revision and **** minor (**** day) revision | ||||
Number of Patient Narratives
|
4 | ||||
| QUALITY ASSURANCE | |||||
Number of Site Audits and Location
|
US, Audit by Sponsor | ||||
Number and Type of Additional Audits
|
n/a | ||||
| TECHNOLOGY | |||||
OmniView / OmniTrack
|
**** months | ||||
| CLINICAL SUPPLIES MANAGEMENT | |||||
Supply, Package, Label, Ship and
Store Study Drug
|
Sponsor | ||||
Provide Randomisation Code
|
Sponsor | ||||
Drug Accountability
|
Sponsor and OMNICARE CR | ||||
Study Drug Disposition, update
Master Drug File
|
OMNlCARE CR | ||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 4
| PROJECT TIMELINE | ||
Protocol Synopsis Finalized
|
**** | |
Final Protocol available and submitted to FDA
|
**** | |
Commencement
of Omnicare Work (Start Date)
|
**** | |
FDA Approval
|
**** | |
Site Initiation
|
**** | |
First Patient Enrolled — cohort 1
|
**** | |
Last Patient Enrolled — cohort 1 (assumes
excluding 21 screening days of final expanded
cohort)
|
**** | |
Last CRF to Data Management Unit.
|
**** | |
Database Lock
|
**** | |
Draft Statistical Analysis
|
**** | |
Final Statistical Analysis
|
**** | |
Draft Clinical Study Report
|
**** | |
Final Clinical Study Report
|
**** | |
Study Completion — End of Omnicare Involvement
|
**** | |
Total Omnicare Involvement
|
**** | |
| Day - **** to day - **** | • | screening for 1st dose cohort | ||||
| Day **** (Baseline) | • | treatment start of 1st dose cohort of treatment arm 1 (single dose application) | ||||
| Day **** | • | DESC (Dose Escalation Steering Committee) evaluation prior to approval to escalate to next dosage concentration | ||||
| • | Treatment start of 2nd dose cohort of treatment arm 1 | |||||
| • | Treatment start of 1st dose cohort of treatment arm 2 (two dose application) | |||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 5
| • | 7 escalations x 2 dose groups of 3 patients x **** days for each DESC review, assumes maximum of **** days. | |
| • | Final Expanded Cohort: **** days screening **** days treatment **** days wound evaluation = assumes maximum of **** days. |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 6
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 7
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 8
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 9
Items discussed at these meetings will include, but will not be limited to:
| • | Therapeutic area and clinical development background | |
| • | Protocol and CRF | |
| • | Discussion of therapeutic implications for the Project | |
| • | Monitoring guidelines | |
| • | Data handling rules from Data Management Plan |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 10
investigators.
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 11
| 1. | Site Selection/ Patient Recruitment Plan |
| 2. | Central Laboratory |
| 3. | Photography of sBCC Lesions |
| 4. | Dermatopathology Laboratory |
| 5. | Setup and Maintenance of Project Master File |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 12
| 6. | Investigator Document Plan |
| 7. | Investigator Essential Document Management |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 13
| 8. | Regulatory and Ethics Committee Submission |
| 9. | Country Specific ICF Adaptations |
| 10. | Facility Letters and other Investigator Notices |
| 11. | Letters of Indemnification |
| 12. | Protocol Amendments and IND Safety Reports and Investigator Brochure Updates |
| 13. | Essential Document Renewals and Updates — Annual Site Maintenance |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 14
| 14. | Clinical Trial Agreements |
| • | Create all clinical study agreements templates and patient budget template with the Sponsor | |
| • | When possible, set up pre-approved alternative language and budget parameters in anticipation of negotiation |
| • | Negotiate all clinical study agreements that satisfy Sponsor requirements on behalf of the Sponsor |
| • | Incorporate changes using pre-approved alternative parameters and if changes are outside parameters, secure approval by the Sponsor |
| • | Obtain signatures from investigators, institutions, facilities, on all clinical study agreements as needed | |
| • | Collect IRS Form W9 and payment information forms from sites |
| • | Track documentation and communication with the sites, Omnicare CR Project Management and the Sponsor. |
| 15. | Clinical Study Agreements Amendments |
| 16. | Vendor/Lab Service Agreements |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 15
| 1. | Clinical Monitoring |
| 2. | Clinical Monitoring Scheme |
| Preparation, | ||||||||||||||||||||
| Total number of | follow-up and | |||||||||||||||||||
| Type of Visit | Visits per site | visits | Time onsite in hours | reporting in hours | Travel time in hours | |||||||||||||||
Qualification Visits |
**** | **** | **** | **** | **** | |||||||||||||||
Initiation Visits |
**** | **** | **** | **** | **** | |||||||||||||||
Interim Monitoring
Visits (frequency
ever 4 weeks) |
**** | **** | **** | **** | **** | |||||||||||||||
Close-out Visits |
**** | **** | **** | **** | **** | |||||||||||||||
| 3. | Site Maintenance |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 16
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 17
| • | Status reports |
| • | Creation of the Data Management Plan |
| • | Database Development |
| • | Set-up of processes |
| • | Training of staff |
| • | Communication between Sponsor and Omnicare |
| • | Working knowledge of all processes |
| • | Central Laboratory Management and Data Loads |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 18
| 1. | Clinical Study Report — Phase III |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 19
| 2. | Narratives |
| 3. | Clinical Writing Input to Statistical Analysis Plan |
| 4. | Compilation of Appendices to Clinical Study Report |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 20
| 1. | Quality Plan |
| 2. | Dermatophatology Laboratory Audit |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 21
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 22
| Omnicare CR, Inc. Clinical Budget for: | ||
Sponsor:
|
Xxxxxx Ltd. | |
Compound:
|
PEP005 topical gel | |
Study:
|
An open-label, multi-center, dose-escalation, cohort
study to determine the maximum tolerated dose and
safety of PEP005 Topical Gel given as either a
single application (on Day 1 = or two applications
(on Day 1 and Day 8) to a superficial Basal cell
carcinoma (sBCC) on the truck |
|
PCN:
|
KO1607 (6 sites, 1 protocol only) | |
| Estimated | ||||||||||||||||||||||||||||
| Pass— | ||||||||||||||||||||||||||||
| Through | Estimated | |||||||||||||||||||||||||||
| Unit Cost | Fess | Costs | Total Cost | |||||||||||||||||||||||||
| Services | Unit | # Units | (USD $) | (USD $) | (USD $) | (USD $) | ||||||||||||||||||||||
| A. Study Management | ||||||||||||||||||||||||||||
| • | Project Director — Americas Assumes ****% FTE (**** days per month) for entire project duration. **** months | Days | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Project Administrative Support/Coordination —Americas (includes support for all functional areas) Assumes ****% FTE (**** days per month) for entire project duration, **** months | Days | **** | **** | **** | **** | **** | |||||||||||||||||||||
| Sub-Total Study Management | **** | **** | **** | |||||||||||||||||||||||||
| B. Clinical Trial Initiation | ||||||||||||||||||||||||||||
| • | Site Screening and Identification Assume to be done by Xxxxxx | |||||||||||||||||||||||||||
| > USA | Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Site Recruitment Assume to be done by Xxxxxx | |||||||||||||||||||||||||||
| > USA | Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Study Master File | All assumes using Omnicare SOPs. | ||||||||||||||||||||||||||
| >Americas: 6 sites for **** months | Site Months | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Regulatory Document Collection | |||||||||||||||||||||||||||
| >USA | Site | **** | **** | **** | **** | |||||||||||||||||||||||
| Central IRB (pass-throughs are estimates only: actual fees will be billed based on specific IRB fees) | Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Protocol Amendments -
excluding ICF change |
|||||||||||||||||||||||||||
| > USA | Amendments/site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Protocol Amendments
— including ICF change |
|||||||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| KO1607 Study PEP005-009 | Page 23 |
| Omnicare CR, Inc. Clinical Budget for: | ||
Sponsor:
|
Xxxxxx Ltd. | |
Compound:
|
PEP005 topical gel | |
Study:
|
An open-label, multi-center, dose-escalation,
cohort study to determine the maximum tolerated
dose and safety of PEP005 Topical Gel given as
either a single application (on Day 1= or two
applications (on Day 1 and Day 8) to a superficial
Basal Cell Carcinoma (sBCC) on the trunk |
|
PCN:
|
KO1607 (6 sites, 1 protocol only) | |
| Estimated | ||||||||||||||||||||||||||||
| Pass- | ||||||||||||||||||||||||||||
| Through | Estimated | |||||||||||||||||||||||||||
| Unit Cost | Fess | Costs | Total Cost | |||||||||||||||||||||||||
| Services | Unit | # Units | (USD $) | (USD $) | (USD $) | (USD $) | ||||||||||||||||||||||
| > USA | Amendments/site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigator Agreement Negotiation — Standard |
|||||||||||||||||||||||||||
| > Americas | Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigator Agreement
Negotiation— Complex |
|||||||||||||||||||||||||||
| > Americas | Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigator Agreement
Amendments — Standard |
|||||||||||||||||||||||||||
| > Americas | Amendments/site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Letters of Indemnification (US
sites only) |
Site | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Facility Letters |
|||||||||||||||||||||||||||
># Standard Facility Letters |
Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
># Complex Facility Letters |
Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Notice Letters |
Site | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | IND Safety Report |
|||||||||||||||||||||||||||
| > USA | Reports/Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigator Brochure Updates |
|||||||||||||||||||||||||||
| > USA | IB Updates/Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Vendor/Lab Service Agreements Americas |
Agreements | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Investigators Meeting
Coordination (pass-through is
for investigator travel
only-other pass-throughs are
indicated below) |
|||||||||||||||||||||||||||
> Coordination Meeting
/US |
Meeting | **** | **** | **** | **** | **** | ||||||||||||||||||||||
>Per Attendee, Meeting / US |
Attendee | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigators’ Meeting
Preparation |
Days | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigators’ Meeting
Attendance (OCR attendees x
days) |
|||||||||||||||||||||||||||
| Project Director — Americas | Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
CTM — Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Data Manager — Int’l |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
|
Medical Monitor — Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
|
Safety Officer — Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| KO1607 Study PEP005-009 | Page 24 |
| Omnicare CR, Inc. Clinical Budget for: | ||
Sponsor:
|
Xxxxxx Ltd. | |
Compound:
|
PEP005 topical gel | |
| An open-label, multi-center, dose-escalation, cohort study to determine the maximum tolerated dose and safety of PEP005 Topical Gel given as either a single application (on Day 1= or two applications (on Day 1 and Day 8) to a superficial Basal Cell Carcinoma | ||
Study:
|
(sBCC) on the trunk | |
PCN:
|
KO1607 (6 sites. 1 prolocol only) | |
| Estimated | ||||||||||||||||||||||||||||
| Past- | ||||||||||||||||||||||||||||
| Through | Estimated | |||||||||||||||||||||||||||
| Unit cost | Costs | Total Cost | ||||||||||||||||||||||||||
| Services | Unit | # Units | (USD $) | Fees (USD $) | (USD $) | (USD $) | ||||||||||||||||||||||
CQA Manager -
Americas
(Optional if
requeued) |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
CRA
Attendees |
||||||||||||||||||||||||||||
> USA:
****CRA |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Client Attendee
Travel |
Attendee | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Sub-Total
Clinical Trial
Initiation |
**** | **** | **** | |||||||||||||||||||||||||
| C | Clinical Trial
Management |
|||||||||||||||||||||||||||
| • | Clinical Trial
Manager -
Americas Assumes ****% (**** days per month) for **** months |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Clinical Monitoring |
|||||||||||||||||||||||||||
> Site
Qualification Visits
Assume not required |
||||||||||||||||||||||||||||
> USA **** hrs on-site
**** hrs for prep, follow-up, and reports, and ****
hrs for travel |
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||||
> Site
Initiation Visits |
||||||||||||||||||||||||||||
> USA **** hrs
on-site, **** hrs
for prep, follow-up, and
reports, and **** hrs for
travel |
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||||
> Site
Initiation Visits |
||||||||||||||||||||||||||||
> USA
assume **** visits/sites **** hrs on site, **** hrs
for prep, follow-up, and
reports, and **** hrs for
travel |
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||||
> Additional Day Interm
Visits (based on **** hour
day, visits are total per
zone, actual, will be
billed) |
||||||||||||||||||||||||||||
> USA: assume **** day for
Zone pool of visits TBD
if required Visits |
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||||
> USA **** hrs on-site ****
hrs for prep follow-up
and reports and
**** hrs for travel |
Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Site Maintance for ****
hrs/month/ site (6 sites
x **** enrollment
and treatment months) |
|||||||||||||||||||||||||||
> USA: 6 sites |
Site Months | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | CRA Monthly
Teleconferences |
|||||||||||||||||||||||||||
> USA **** CRA |
Months | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 25
| Omnicare CR, Inc. Clinical Budget for: | ||
Sponsor:
|
Xxxxxx Ltd. | |
Compound:
|
PEP005 topical gel | |
Study:
|
An open-label, multi-center, dose-escalation, cohort study to determine the maximum tolerated dose and safety of PEP005 Topical Gel given as either a single application (on Day 1 = or two applications (on Day 1 and Day 8) to a superficial Basal Cell Carcinoma (sBCC) on the trunk | |
PCN:
|
KO1607 (6 sites. 1 protocol only) | |
| Estimated Pass — | ||||||||||||||||||||||||||||
| Through Costs | Estimated Total | |||||||||||||||||||||||||||
| Services | Unit | # Units | Unit Cost (USD $) | Fees (USD $) | (USD $) | Cost (USD $) | ||||||||||||||||||||||
| • | Project Team Training |
|||||||||||||||||||||||||||
Project Director — America |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
CTM — Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Data Manager — Int’l |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Medical Monitor — America |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Safety Officer — Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Statistrician — Int’l |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
CRA Attendees |
Days | |||||||||||||||||||||||||||
> USA CRAs; **** CRA |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Sponsor Meeting
Attendance (xxxx on
actual) |
|||||||||||||||||||||||||||
Project Director — Americas Assumes **** TC , **** hours |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
|
CTM — Americas Assume, **** TC **** hours |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
|
Data Manager — Int’l |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Medical Monitor — Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Safety Officer — Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
|
Statistrician — Int’l |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
|
St. Writer —
Americas |
Days | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Clinical Grants
Administration |
|||||||||||||||||||||||||||
|
> Grant Payment
Set-up |
||||||||||||||||||||||||||||
|
> USA |
Sites | **** | **** | **** | **** | |||||||||||||||||||||||
|
> Grant
Management (takes place for active site from site
set-up through **** days after sites close-out; |
||||||||||||||||||||||||||||
|
estimated amounts,
actuals will be
billed based on
number of active
sites set up) |
||||||||||||||||||||||||||||
|
> USA |
Site Quarters | **** | **** | **** | **** | |||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
|
> Payment
Processing (estimated based on **** payments/site;
actuals, including investigator and site related
payment and any miscellaneous payments, actuals
will be billed, pass through costs are related to
photocopying and postage) |
||||||||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 26
| Omnicare CR, Inc. Clinical budget for: | ||||
| Sponsor: | Xxxxxx Ltd. | |||
| Compound: | PEP005 topical gel | |||
| An open-label, multi-center, dose-escalation, cohort study to determine the maximum tolerated dose and safety of PEP005 Topical Gel given as either a single application (on Day 1= or two applications (on Day 1 and Day 8) to a superficial Basal Cell Carcinoma | ||||
| Study: | (sBCC) on the trunk | |||
| PCN: | KO1607 (6 sites, 1 protocol only) | |||
| Estimated | ||||||||||||||||||||||||
| Pass - | ||||||||||||||||||||||||
| Through | Estimated | |||||||||||||||||||||||
| Unit Cost | Costs | Total Cost | ||||||||||||||||||||||
| Services | Unit | # Units | (USD $) | Fees (USD $) | (USD $) | (USD $) | ||||||||||||||||||
| > USA 6 sites | Payments | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Estimated
Investigator Grants |
|||||||||||||||||||||||
Assumes one
follow-up visit per
patient, no
Academic Medical
Centers
|
Enrolled Patient — Americas | **** | **** | **** | **** | |||||||||||||||||||
| • | Refund Checks (if
needed, actuals
will be billed)
|
Refund | **** | **** | **** | **** | **** | |||||||||||||||||
Sub Total clinical
Trial Management
|
**** | **** | **** | |||||||||||||||||||||
| D. Safety Medical | ||||||||||||||||||||||||
| • | Medical Monitoring
availability during
business hours
(flat fee of ****
hours per month
from first patient
in through last
patient out, ****
months) Covers both
studies |
|||||||||||||||||||||||
> Americas
|
Month | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Medical Monstoring
during Study Start
Up and Study
Close-out (Billed
on actuals) |
|||||||||||||||||||||||
> Americas
|
Hours | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Safety Coordinator |
|||||||||||||||||||||||
>
Americas —
assumes **** days
per month for ****
months + **** days for
safety plan
generation
|
Days | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Availability for
receipt of SAEs
during non-business
hours (first
patient in to last
patient out plus
one month) |
|||||||||||||||||||||||
> Americas
|
Months | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | SAE Reporting
(without
narratives) Billed
on actuals
|
Report | **** | **** | **** | **** | **** | |||||||||||||||||
| • | SAE Reporting (with
initial descriptive
summary) Billed on
actuals
|
Report | **** | **** | **** | **** | **** | |||||||||||||||||
| • | SAE Reporting
(Follow-up/Revision
Reports > ****)
Billed on actuals
|
Report | **** | **** | **** | **** | **** | |||||||||||||||||
| • | **** |
|||||||||||||||||||||||
> Project Set-Up
Assume not required
as database for
xxxxxx already
exists at Omnicare
|
Project | **** | **** | **** | **** | **** | ||||||||||||||||||
> Monthly
Database
Maintenance (first
patient
|
Month | **** | **** | **** | **** | **** | ||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 27
| Omnicare CR, Inc. Clinical Budget for: | ||||
| Sponsor: | Xxxxxx Ltd. | |||
| Compaound: | PEP005 topical gel | |||
| An open-label, multi-center, dose-escalation, cohort study to determine the maximum tolerated dose and safety of PEP005 Topical Gel given as either a single application (on Day 1 = or two applications (on Day 1 and Day 8) to a superficial Basal Cell Carcinoma | ||||
| Study: | (sBCC) on the trunk | |||
| PCN: | KO1607 (6 sites, 1 protocol only) | |||
| Estimated | ||||||||||||||||||||||||
| Pass - | ||||||||||||||||||||||||
| Through | Estimated | |||||||||||||||||||||||
| Unit Cost | Costs | Total Cost | ||||||||||||||||||||||
| Services | Unit | # Units | (USD $) | Fees (USD $) | (USD $) | (USD $) | ||||||||||||||||||
in to database close)
Covers both studies |
||||||||||||||||||||||||
> Data Entry into
**** (actuals will be
billed)
|
SAE | **** | **** | **** | **** | **** | ||||||||||||||||||
Sub-Total Safety and
Medical
|
**** | **** | **** | |||||||||||||||||||||
| E. Clinical Data Management | ||||||||||||||||||||||||
| • | Clinical Data
Management Oversight —
int’l (CDM Manager
assumes ****% FTE x ****
months for Start-up.
****% FTE for ****
months for Duration and
****% FTE for **** months
for Close-out)
|
Days | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Lead CDA — Int’l
(assumes ****% FTE x ****
months, ****% FTE x ****
months, and ****% FTE
for **** months)
|
Days | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Development of Data
Management Plan
(includes **** major and ****
minor revision; add’l
revisions will be
billed at ****
rates)
|
Days | **** | **** | **** | **** | **** | |||||||||||||||||
| • | CRF Design (assumes ****
page CRF, **** unique CRE
pages); includes ****
major and **** minor
revision
|
Project | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Database Development,
Testing and Maintenance
(assumes **** page CRF,
**** unique CRF pages)
|
Project | **** | **** | **** | **** | **** | |||||||||||||||||
| • | Estimated Data Entry (actuals will be billed) |
|||||||||||||||||||||||
> Pages in Int’l
|
Pages | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Estimated Data Review
and Query Resolution
(assumes **** issue per ****
CRF pages, **** manual
checks and the
application of **** study
assumption for **** CRF
pages); assumes **** CRF
pages per enrolled
patient, **** pages per
screen failure patient,
and **** pages per drop
out patient. Queries
will be billed on
actuals. |
|||||||||||||||||||||||
> CRF Pages
|
Page | **** | **** | **** | **** | **** | ||||||||||||||||||
| • | Quality Control Check of
|
Patients | **** | **** | **** | **** | **** | |||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 28
Sponsor:
|
Xxxxxx Ltd. | |
Compound:
|
PEP005 topical gel | |
| An open-label, multi-center, dose-escalation, cohort study to determine the maximum tolerated dose and safety of PEP005 Topical Gel given as either a single application (on Day 1 = or two applications (on Day land Day 8) to a superficial Basal Cell Carcinoma | ||
Study: |
(sBCC) on the trunk | |
PCN:
|
KOI607 (6 sites. 1 protocol only) |
| Estimated | ||||||||||||||||||||||||||||
| Pass | ||||||||||||||||||||||||||||
| Trough | Estimated | |||||||||||||||||||||||||||
| Unit Cost | Cost | Total Cost | ||||||||||||||||||||||||||
| Services | Unit | # Units | (USD $) | Fees (USD $) | (USD $) | (USD $) | ||||||||||||||||||||||
| Database versus CRF (assumes **** CRF pages per patient) QC of critical variables, assume **** min per patient, **** patients | ||||||||||||||||||||||||||||
| • | Quality Control Check of Database versus CRF (assumes **** CRF pages per patient) Full QC check, assume **** min per patient, **** patients | Patients | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Edit Development (actuals will be billed) | Edits | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | CRF and Query Tracking (includes all ancillary Pages; actuals will be billed) Assumes **** min per page | Page | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Concomitant Medication ATC Coding (actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Dictionary Coding of Adverse Event terms to MedDRA (estimated to be **** per patient actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Dictionary Coding of Medication Terms (estimated to be **** per patient actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Central Laboratory—Initial Load (actual will be billed) | |||||||||||||||||||||||||||
| Initial Load | Load | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| >Subsequent Load (actuals will be billed) Assumes monthly during **** months enrolment + treatment period to database lock | Monthly | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| Lab Visit Verification (**** visits x **** patients; actuals will be billed) | Visits | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Reconciliation of the Safety and Clinical Database (actuals will be billed) | SAE | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Protocol deviation log Assumes ****hrs of Programmer time | Hours | **** | **** | **** | **** | ||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 29 |
| Omnicare CR, Inc. Clinical Budject for: | ||
Sponsor:
|
Xxxxxx Ltd. | |
Compound:
|
PEP005 topical gel | |
| An open-label, multi-center, dose-escalation, cohort study to determine the maximum tolerated dose and safety of PEP005 Topical Gel given as either a single application (on Day 1 = or two applications (on Day 1 and Day 8) to a superficial Basal Cell Carcinoma | ||
Study:
|
(sBCC) on the trunk | |
PCN:
|
KO1607 (6 sites. 1 protocol only) | |
| Estimated | ||||||||||||||||||||||||||||
| Past- | ||||||||||||||||||||||||||||
| Through | Estimated | |||||||||||||||||||||||||||
| Unit Cost | Cost | Total Cost | ||||||||||||||||||||||||||
| Services | Unit | # Units | (USD $) | Fees (USD $) | (USD $) | (USD $) | ||||||||||||||||||||||
| • | DESC Meeting -Customized Reports | Report | **** | **** | **** | **** | **** | |||||||||||||||||||||
| Sub-Total Clinical Data Management | **** | **** | **** | |||||||||||||||||||||||||
| F | . | Biometrics Analysis | ||||||||||||||||||||||||||
| • | Biometrics Team Manager Assumes **** days per month for **** months | Days | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Project Data Setup |
Project | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Statistical Analysis Plan | Project | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Design of Table Shells (Mocks) | Project | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Programming/QC of Data Displays (actuals will be billed). | |||||||||||||||||||||||||||
| Unique Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| Repeat Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| Unique Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| Repeat Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| Unique Figures | Figure/Graph | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Repeat Figures |
Figure/Graph | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Statistical Analysis |
|||||||||||||||||||||||||||
>Regular |
Project | **** | **** | **** | **** | **** | ||||||||||||||||||||||
| • | FDA Item 11 |
Project | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Standard Data
Transfer |
|||||||||||||||||||||||||||
>Initial |
Transfer | **** | **** | **** | **** | **** | ||||||||||||||||||||||
>Subsequent |
Transfer | **** | **** | **** | **** | **** | ||||||||||||||||||||||
>Add **** datasets
will be billed @
$**** Euro
per dataset |
||||||||||||||||||||||||||||
| Add 1 Stats consulting meeting attendance, etc., will be charged at **** rates as follows: | ||||||||||||||||||||||||||||
Team
Leader |
Days | **** | ||||||||||||||||||||||||||
Statistician |
Days | **** | ||||||||||||||||||||||||||
Programmer |
Days | **** | ||||||||||||||||||||||||||
Sub-Total
Biometrics |
**** | **** | **** | |||||||||||||||||||||||||
| G. | Clinical Writing | All assumes using Omnicare SOPs. | ||||||||||||||||||||||||||
| • | Clinical Study
Report |
|||||||||||||||||||||||||||
>Phase 11/111
Report
(includes **** major
(up to
**** days) and ****
minor (up
to **** day revision) |
Report | **** | **** | **** | **** | **** | ||||||||||||||||||||||
> Tables
(greater than ****) |
Report | **** | **** | **** | **** | |||||||||||||||||||||||
| • | Narratives (actuals
will be billed) |
Narrative | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Clinical Writing
input to |
Project | **** | **** | **** | **** | **** | |||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 30 |
| Omnicare CR, Inc. Clinical Budget for: | ||||
| Sponsor: | Xxxxxx Ltd. | |||
| Compound: | PEP005 topical gel | |||
| An open-label, multi-center, dose-escalation, cohort study to determine the maximum tolerated dose and safety of PEP005 Topical Gel given as either a single application (on Day 1 = or two applications (on Day 1 and Day 8) to a superficial Basal Cell Carcinoma | ||||
| Study: | (sBCC) on the trunk | |||
| PCN: | KO1607 (6 sites. 1 protocol only) | |||
| Estimated | ||||||||||||||||||||||||||||
| Pass — | ||||||||||||||||||||||||||||
| Through | ||||||||||||||||||||||||||||
| Unit Cost | costs | Estimated Total | ||||||||||||||||||||||||||
| Services | Unit | # Units | (USD $) | Fees (USD $) | (USD$) | Cost (USD $) | ||||||||||||||||||||||
SAP |
||||||||||||||||||||||||||||
| Attendance at Sponsor Requested
Meetings: Attendance at Sponsor requested meetings (teleconferences/video conferences or client review/planning meetings at Sponsor/Omnicare CR, Inc ) will be billed to Sponsor according to the following per diem rates |
||||||||||||||||||||||||||||
Director: |
$ | **** | ||||||||||||||||||||||||||
Senior Writer |
$ | **** | ||||||||||||||||||||||||||
Clinical Writer: |
$ | **** | ||||||||||||||||||||||||||
| Sponsor will be billed for actual time expended | ||||||||||||||||||||||||||||
Sub-Total Clinical Writing |
**** | **** | **** | |||||||||||||||||||||||||
| H. Technology | ||||||||||||||||||||||||||||
| • | Omni View |
|||||||||||||||||||||||||||
>Set-up |
Site | **** | **** | **** | **** | **** | ||||||||||||||||||||||
>Monthly
Maintenance |
Months | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Sub-Total Technology |
**** | **** | **** | |||||||||||||||||||||||||
| 1. Regulatory Services | ||||||||||||||||||||||||||||
| • | CRF Filing and
Reconciliation (xxxx
on actuals) |
Pages | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Return of CRF (hard
copy.); xxxx on
actuals |
Page | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Return of Investigator
and Study-Wide Documents (paper) |
Sites | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Report SAEs
to FDA Agency |
# SAEs | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Compilation of
Clinical Study Report
Appendices (electronic
copy) |
|||||||||||||||||||||||||||
> Setup and
Management Fee |
Report | **** | **** | **** | **** | **** | ||||||||||||||||||||||
>
Scanning, actuals
will be billed |
Pages | **** | **** | **** | **** | **** | ||||||||||||||||||||||
> Volumes (fee does
not include
hyper-linking): actuals will be billed |
Volume | **** | **** | **** | **** | **** | ||||||||||||||||||||||
Sub-Total Regulatory
Services |
**** | **** | **** | |||||||||||||||||||||||||
| J. Clinical Quality Assurance | ||||||||||||||||||||||||||||
| • | Quality Plan |
Days | **** | **** | **** | **** | **** | |||||||||||||||||||||
| • | Contract Provider
Audits
(****)
(fees include
preparation, travel,
on-site and reporting
time) |
Site | **** | **** | **** | **** | **** | |||||||||||||||||||||
Sub-Total Clinical |
**** | **** | **** | |||||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 31
| Omnicare CR, Inc. Clinical Budget for: | ||||
| Sponsor: | Xxxxxx Ltd. | |||
| Compound: | PEP005 topical gel An open-label, multi-center, dose-escalation, cohort study to determine the maximum tolerated dose and safety of PEP005 Topical Gel given as either a single application (on Day 1 = or two applications (on Day 1 and Day 8) to a superficial Basal Cell Carcinoma |
|||
| Study: | (sBCC) on the trunk | |||
| PCN: | KO1607 (6 sites. 1 protocol only) | |||
| Estimated Pass- | ||||||||||||||||||||||||||||
| Through Costs | Estimated Total | |||||||||||||||||||||||||||
| Services | Unit | # Units | Unit Cost (USD $) | Fees (USD $) | (USD $) | Cost (USD $) | ||||||||||||||||||||||
Quality Assurance |
||||||||||||||||||||||||||||
| L. Miscellaneous Pass-Through Expenses | ||||||||||||||||||||||||||||
| • | CRF Printing (xxxx on actuals) Assume
one CRF per
subject + ****% extra |
Pages | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Investigator
Binders (includes printing, copying and shipping) Assume one per investigator |
Binder | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Shipment of Start-up
Packet (**** shipment x 6 sites) |
Sites | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Phone Center |
Month | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Beeper/Pager(# x
months) |
Month | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Dedicated Fax Line |
Month | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Central Lab - **** |
Project | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Photography Lab
Assume at least one picture of selected **** at each visit cameras for 6 sites |
Project | **** | **** | **** | **** | ||||||||||||||||||||||
| • | Dermatopathology
Lab - **** **** |
Project | **** | **** | **** | **** | ||||||||||||||||||||||
Sub-Total Miscellaneous |
**** | **** | ||||||||||||||||||||||||||
Pass Through Expenses |
||||||||||||||||||||||||||||
| Estimated Services Budget | **** | |||||||||||||||||||||||||||
| Estimated Pass Through | **** | |||||||||||||||||||||||||||
| Total Estimated Budget | **** | |||||||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 32
| • | Travel | ||
| • | Delivery Costs | ||
| • | CRF and other printing or copying costs | ||
| • | Investigator Meeting costs | ||
| • | Telecommunication Costs (which may include telephone, fax, paper, conference calls, or PC connectivity charges) | ||
| • | All other Project related expenses that are not related to service fees |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 33
BY AND BETWEEN: |
||||
Xxxxxx Operations Pty Ltd.
|
Omnicare CR, Inc. | |||
BY:
/s/ Xxxxx Xxxxxxx
|
BY: /s/ Xxxx Xxxxx | |||
Name:
Xxxxx Xxxxxxx Title: General Manager Australia Dated: 15/1/07 |
Name: Xxxx Xxxxx Title: CEO Dated: 02/21/07 |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 34
between Xxxxxx Operations Pry Ltd. and Omnicare CR, Inc.,
dated 1st June 2005
| I. | Project Plan |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| III. | Omnicare CR Services |
| Task List (Major) | Xxxxxx | Omnicare | ||
Investigator’s Meeting |
||||
1. Plan Investigator’s Meeting
|
ü | |||
2. Prepare binders for the meeting
|
ü | |||
3. Present Investigator’s meeting
|
ü | |||
4. Attendance at meeting — Qld. — Biostatistician Only
|
ü | ü | ||
Medical Management |
||||
1. Safety Plan and Master File Set Up
|
ü | |||
2. Document and manage all SAEs
|
ü | |||
3. Cover medical emergencies after hours (pager)
|
ü | |||
4. Develop and maintain safety database
|
ü | |||
5. Submit SAE reports to regulatory authorities
|
ü | |||
6. Prepare safety updates
|
ü | |||
7. Medical Monitor- Review of SAEs
|
ü | |||
Data Entry |
||||
1.
Design/develop data collection system
|
ü | |||
2. Validate data collection system
|
ü | |||
3.
Document control of CRFs
|
ü | |||
4. Enter and verify da,ta
|
ü | |||
Data Management |
||||
1. Design/develop data cleaning system
|
ü | |||
2 Validate cleaning system
|
ü | |||
3. Write data management guidelines and edit
specifications
|
ü | |||
4. Write CRF completion guidelines
|
ü | |||
5. Review CRF and run edit system
|
ü | |||
6. Resolve edit queries
|
ü | |||
7. Incorporate laboratory data into database
|
ü | |||
8. Document corrections to CRFs
|
ü |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 2
| Task List (Major) | Xxxxxx | Omnicare | ||
Date Management (continued) |
||||
9. Perform QC audits — electronic data vs paper CRFs
|
ü | |||
10. Code adverse events
|
ü | |||
11. Code medications (terms and ATC)
|
ü | |||
12. Test data transfer after 25% of patients completed
|
ü | |||
13. Test data transfer after 75% of patients completed
|
ü | |||
14. Produce protocol deviation logs
|
ü | |||
15. Provide DESC data listings
|
ü | |||
Biometrics |
||||
1. Prepare a statistical analysis plan prior to
database lock
|
ü | |||
2. CRF development and statistical review
|
ü | |||
3. Define efficacy tables and listings
|
ü | |||
4. Define safety tables and listings
|
ü | |||
5. Produce efficacy tables and listings
|
ü | |||
6. Produce safety tables and listings
|
ü | |||
7. Provide draft report templat and analysis plan
|
ü | |||
8. Approve report template
|
ü | |||
9. Validate efficacy tables and listings
|
ü | |||
10. Validate safety tables and listings
|
ü | |||
11. Perform quality assurance audit of the tables and
listings
|
ü | |||
12. Provide final tables and listings
|
ü | |||
13. Provide statistical analysis
|
ü | |||
Report Preparation |
||||
1.
Prepare draft report template
|
ü | |||
2. Approve final report template
|
ü | |||
3. Draft study report
|
ü | |||
4. Final study report
|
ü | |||
5. Perform quality assurance of study report
|
ü | |||
6. Approval of final study report
|
ü | |||
7. Database transfer to Client
|
ü |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
Page 3
| Activities | Anticipated Timeline | |
Project Start Up
|
**** | |
First Patient In
|
**** | |
Last Patient In (assumes **** months recruitment period)
|
**** | |
Last Patient Out (assumes **** months treatment time)
|
**** | |
Last CRF to Data Management
|
**** | |
Database Close
|
**** | |
Statistical Analysis Available
|
**** | |
Clinical Study Report provided to Xxxxxx
|
**** | |
Project Close-out
|
**** | |
Total Project Duration
|
**** |
| V. | Budget |
| A. | Estimated Project Budget |
| # | Estimated | Estimated | ||||||||||||||||||||||
| Services | Unit | Units | Unit Cost | Fees | Pass-Thru | Total Cost | ||||||||||||||||||
| A. Study Management and Investigator Meeting Attendance | ||||||||||||||||||||||||
•
|
Project Administrative Support/Coordination — Int’l (incl. support for all functional areas) for project duration of **** months | Days | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Investigators’ Meeting Attendance (Statistician — Australia) | Days | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Study Management and Investigator Meeting Attendance | **** | **** | **** | |||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 4 | ||||||
| # | Estimated | Estimated | ||||||||||||||||||||||
| Services | Unit | Units | Unit Cost | Fees | Pass-Thru | Total Cost | ||||||||||||||||||
| B. Safety and Medical | ||||||||||||||||||||||||
•
|
Medical Monitoring availability during business hours (flat fee of **** hours per month from first patient in through last patient out) | Months | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Medical Monitoring availability during non-business hours (from first patient in through last patient out; billed on actuals) | Hours | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Safety Coordinator (assumes **** day/month for **** months |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
SAE Reporting (with initial descriptive summary) Billed on actuals |
Report | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
SAE Reporting (Follow-up/Revision Report >****) Billed on actuals |
Report | **** | **** | **** | **** | **** | |||||||||||||||||
| • | **** | |||||||||||||||||||||||
| >Monthly Database Maintenance (first patient in to database close) | Month | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Data Entry into **** (actuals will be billed) |
SAE | **** | **** | **** | **** | **** | ||||||||||||||||||
•
|
Safety Reporting to Regulatory Authorities — Int’l (assumes **** estimated **** hours per reportable SAE (‘SUSAR’); actuals will be billed | Hours | **** | **** | **** | **** | **** | |||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 5 |
| # | Estimated | Estimated | ||||||||||||||||||||||
| Services | Unit | Units | Unit Cost | Fees | Pass-Thru | Total Cost | ||||||||||||||||||
•
|
Safety Reporting of SAEs to Investigator Sites — Int’l (estimated **** hours per reportable SAE (‘SUSAR’); actuals will be billed) | Hours | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Safety Plan and Master File Set Up (also includes protocol familiarisation) | Project | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Safety and Medical | **** | **** | **** | |||||||||||||||||||||
| C. Clinical Data Management | ||||||||||||||||||||||||
•
|
Clinical Data Management Oversight — Int’l (CDM Manager assumes ****% FTE x **** months for Start- up, ****% FTE for **** months for Duration, and ****% FTE for **** months for Close-out) | Days | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Lead CDA — Int’l (assumes ****% FTE x **** months. ****% FTE x **** months, and ****% FTE for **** months | Days | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Development of Data Management Plan (includes **** major and **** minor revision; add’l revisions will be billed at per diem rates) | Days | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
CRF Design (assumes **** page CRF, **** unique CRF pages); includes **** major and **** minor revision | Project | **** | **** | **** | **** | **** | |||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 6 |
| # | Estimated | Estimated | ||||||||||||||||||||||
| Services | Units | Units | Unit Cost | Fees | Pass-Thru | Total Cost | ||||||||||||||||||
•
|
Database Development, Testing and Maintenance (assumes **** page CRF, **** unique CRF | Project | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Estimated Data Entry (actuals will be billed) | Pages | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Estimated Data Review and Query Resolution (assumes **** issue per **** CRF pages, **** manual checks and the application of **** study assumption for: **** CRF pages); assumes **** CRF pages per enrolled patient, **** pages per screen failure patient, and **** pages per drop out patient. Queries will be billed on actuals. | Pages | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Quality Control Check of Database versus CRF (QC of critical variables) | Patients | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Quality Control Check of Database versus CRF (assumes **** CRF pages per patient); Full QC check) | Patients | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Edit, Development (actuals will be billed) |
Edits | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
CRF and Query Tracking (includes all ancillary pages; actuals will be billed) | Pages | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Dictionary Med remapping — ATC Meds (actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 7 |
| # | Estimated | Estimated | ||||||||||||||||||||||
| Services | Units | Units | Unit Cost | Fees | Pass-Thru | Total Cost | ||||||||||||||||||
•
|
Dictionary Coding of Adverse Event terms to MedDRA (estimated to be **** per patient; actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Dictionary Coding of Medication Terms (estimated to be **** per patient; actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
External Vendor — Initial Load (actuals will be billed) | |||||||||||||||||||||||
| > Initial Load | Load | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Subsequent Load (actuals will be billed) | Load | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Lab Visit Verification (**** visits x **** patients; actuals will be billed) | Visits | **** | **** | **** | **** | **** | ||||||||||||||||||
•
|
Reconciliation of the Safety and Clinical Database (actuals will be billed) | SAE | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Protocol Deviation Log Load | Load | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
DESC data listings (**** DESC reports at **** hours programmer time per report) | Report | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
CRF Filing and Reconciliation (xxxx on actuals) | Pages | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Return of CRF (hard copy); xxxx on actuals | Pages | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Clinical Data Management | **** | **** | **** | |||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 8 |
| # | Estimated | Estimated | ||||||||||||||||||||||
| Services | Units | Units | Unit Cost | Fees | Pass-Thru | Total Cost | ||||||||||||||||||
| D. Biometrics Analysis | ||||||||||||||||||||||||
•
|
Biometrics Team Manager | Days | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Project Data Setup | Project | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Statistical Plan | Project | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Randomisation Schedule |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Design of Table Shells (Mocks) | Project | **** | **** | **** | **** | **** | |||||||||||||||||
| > Unique Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||
| > Repeat Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||
| > Unique Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||
| > Repeat Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||
•
|
Programmatic Evaluability/Outcome |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Statistical Analysis | Project | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
FDA Item 11 | Project | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Standard Data Transfer (initial only) |
Transfer | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Biometrics review of CRF | Hours | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Biometrics Analysis | **** | **** | **** | |||||||||||||||||||||
| E. Clinical Writing | ||||||||||||||||||||||||
•
|
Clinical Study Report | |||||||||||||||||||||||
| > Phase II/III Report (includes **** major (up to **** days) and **** minor (up to **** day) revision) | Report | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Tables (greater than ****) |
Report | **** | **** | **** | **** | **** | ||||||||||||||||||
•
|
Narratives (actuals will be billed) |
Narrative | **** | **** | **** | **** | **** | |||||||||||||||||
•
|
Clinical Writing Input to SAP | Project | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Clinical Writing | **** | **** | **** | |||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 9 |
| # | Estimated | Estimated | ||||||||||||||||||||||
| Services | Units | Units | Unit Cost | Fees | Pass-Thru | Total Cost | ||||||||||||||||||
| Estimated Services Budget | **** | |||||||||||||||||||||||
| Estimated Pass Through | **** | |||||||||||||||||||||||
| Total Estimated Budget | **** | |||||||||||||||||||||||
| B. | Payment Schedules | |
| 1. | Invoicing Process for Service Fees |
| 2. | Exchange Rates |
| 3. | Pass-Through Expense Invoicing |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 10 |
| • | Travel | ||
| • | Delivery Costs | ||
| • | Printing or copying costs | ||
| • | Meeting costs | ||
| • | Telecommunication Costs (which may include telephone, fax, paper, conference calls, or PC connectivity charges) | ||
| • | All other Project related expenses that are not related to service fees |
Westpac Banking Corporation,
Xxxxxxxx Xxxxxxxx Xxxxxx,
Xxxxx Xxxx, XXX
SWIFT ID: ****
BSB/Account Number: ****
| BY AND BETWEEN: | ||||||||||||||
| Xxxxxx Operations Pty Ltd. | Omnicare CR, Inc. | |||||||||||||
BY:
|
/s/ XXXXX XXXXXXX | BY: | /s/ Xxxx Xxxxx | |||||||||||
| Name: | XXXXX XXXXXXX | Name: | Xxxx Xxxxx | |||||||||||
| Title: | GENERAL MANAGER, AUS. | Title: | CEO | |||||||||||
| Date: | 14 FEBRUARY 2007 | Date: | 03/05/07 | |||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 11 |
between Xxxxxx Operations Pty Ltd. and Omnicare CR, Inc.,
dated 1st June 2005
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 1 |
| Scenario 1 - US | ||||
| Task List (Major) | Xxxxxx | Omnicare | ||
CT Approvals |
||||
1. Submit regulatory documents to relevant authorities |
ü | |||
2. Prepare and submit Ethics Committee Applications |
ü | |||
Investigator’s Brochure Preparation — to be ready 10 September |
ü | |||
1. Protocol development (literature review, background) |
ü | |||
2. Design and write protocol |
ü | |||
3. Approve protocol |
ü | |||
4. Print and bind protocol |
ü | |||
5. Distribute protocol to sites |
ü | |||
6. Investigator Drug Brochure Preparation |
ü | |||
7. Draft prototype informed consent |
ü | |||
8. Approve prototype informed consent |
ü | |||
CRF Preparation |
||||
1. Design and draft CRFs |
ü | |||
2. Provide input into the development of the CRF, as reqd |
ü | |||
3. Approve CRFs |
ü | |||
4. Print and assemble CRFs |
ü | |||
5. Distribute CRFs to sites |
ü | |||
6. Write CRF conventions guide |
ü | |||
Project Management |
||||
1. Provide weekly enrolment updates |
ü | |||
2. Regular update of cumulative monitoring visit schedule |
ü | |||
3. Provide weekly updates of CRFs status (received, data
entered, cleaned and number of queries outstanding) |
ü | |||
4. Team meetings with minutes |
ü | |||
5. Provision of status reports to clients of performance
against deliverables. |
ü | |||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 2 |
| Scenario 1 - US | ||||
| Task List (Major) | Xxxxxx | Omnicare | ||
Qualification visits |
||||
1. Develop list of potential investigators |
ü | |||
2. Screen Investigators via surveys/telephone interviews |
ü | |||
3. Conduct site qualification visit, if applicable |
ü | |||
4. Provide written site evaluation report |
ü | |||
5. Prepare Investigator contract |
ü | |||
6. Negotiate Investigator grants |
ü | |||
Pre-Study Activities |
||||
1. Collect all regulatory documents from each site |
ü | |||
2. Select central laboratory |
N/A | |||
3. Select drug packaging facility |
N/A | |||
4. Select central ethics committee (if applicable) |
N/A | |||
5. Prepare study file notebooks for sites |
ü | |||
6. Generate monitoring plan |
ü | |||
7. Generate data entry/management plan |
ü | |||
8. Set up project master files |
ü | |||
Investigator’s Meeting (if applicable) |
||||
1. Plan Investigator’s Meeting |
N/A | |||
2. Prepare binders for the meeting |
N/A | |||
3. Present Investigator’s meeting |
N/A | |||
4. Attendance at meeting |
N/A | |||
Initiation Visits |
||||
1. Conduct site initiation visits |
ü | |||
2. Provide site initiation report |
ü | |||
On-Site Monitoring |
||||
1. Conduct monitoring visits |
ü | |||
2. Provide site monitoring reports |
ü | |||
3. Verify 100% of source documentation |
ü | |||
4. Review drug records |
ü | |||
5. Review lab storage |
N/A | |||
6. Review monitoring and data retrieval plan |
ü | |||
7. Resolve data queries as they arise |
ü | |||
Close-out visits |
||||
1. Conduct site close-out visit |
ü | |||
2. Provide close-out trip report |
ü | |||
3. Prepare study documents for archiving |
ü | |||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 3 |
| Scenario 1 - US | ||||
| Task List (Major) | Xxxxxx | Omnicare | ||
Site Management |
||||
1. Manage all site questions and prepare a Q&A document |
ü | |||
2. Pay investigators (excludes PTCs for grant payments) |
ü | |||
3. Pay drug packaging facility |
ü | |||
4. Pay central laboratory |
N/A | |||
5. Maintain weekly telephone contact log with site |
ü | |||
Interactive Voice Response System (IVRS) |
||||
1. Provision of IVRS randomisation and blinding/
unblinding |
N/A | |||
Medical Management |
||||
1. Document and manage all SAEs |
ü | |||
2. Cover medical emergencies after hours (pager) |
ü | |||
3. Develop/ maintain safety database |
ü | |||
4. Submit SAE reports to regulatory authorities |
ü | |||
5. Prepare safety updates |
ü | |||
6. Medical Monitor — Review of SAEs |
ü | |||
Study Drug Management |
ü | |||
Data Entry |
||||
1. Design/develop data collection system |
ü | |||
2. Validate data collection system |
ü | |||
3. Document control of CRFs |
ü | |||
4. Enter and verify data |
ü | |||
Data Management |
||||
1. Design/develop data cleaning system |
ü | |||
2. Validate cleaning system |
ü | |||
3. Write data management guidelines and edit
specifications |
ü | |||
4. Review CRF and run edit system |
ü | |||
5. Resolve edit queries |
ü | |||
6. Incorporate laboratory data into database |
N/A | |||
7. Document corrections to CRFs |
ü | |||
8. Perform QC audits — electronic data compared to
paper CRFs |
N/A | |||
9. Code adverse events |
ü | |||
10. Code medications |
ü | |||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 4 |
| Scenario 1 - US | ||||
| Task List (Major) | Xxxxxx | Omnicare | ||
Statistical Analysis |
||||
1. Prepare a statistical analysis plan prior to
CRF finalisation |
ü | |||
2. Review of protocol statistical methods |
ü | |||
3. CRF development and statistical review |
ü | |||
4. Define efficacy tables and listings |
ü | |||
5. Define safety tables and listings |
ü | |||
6. Produce efficacy tables and listings |
ü | |||
7. Produce safety tables and listings |
ü | |||
8. Provide draft report template and analysis plan |
ü | |||
9. Approve report template |
ü | |||
10. Validate efficacy tables and listings |
ü | |||
11. Validate safety tables and listings |
ü | |||
12. Perform quality assurance audit of the tables
and listings |
ü | |||
13. Provide final tables and listings |
ü | |||
14. Provide statistical study report |
N/A | |||
Report Preparation |
||||
1. Prepare draft report template |
ü | |||
2. Approve final report template |
ü | |||
3. Draft study report |
ü | |||
4. Final study report |
ü | |||
5. Perform quality assurance of study report |
ü | |||
6. Approval of final study report |
ü | |||
7. Top Line report from Day 29 information |
ü | |||
8. Database transfer to Client |
ü | |||
Regulatory Site Audits |
ü | |||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 5 |
PROJECT TIMELINE |
||
Commencement of Work (Start Date)
|
**** | |
Protocol Finalised
|
**** | |
First Patient Enrolled
|
**** | |
Last Patient Enrolled
|
**** | |
Last Patient End of Treatment
|
**** | |
Last CRF to Data Management
|
**** | |
Database Lock
|
**** | |
Statistical Analysis
|
**** | |
Study End
|
**** | |
Study Completion — End Omnicare Involvement
|
**** |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 6 |
| Omnicare CR, Inc. Clinical Budget for: Sponsor: Compound: Study: PCN: |
Xxxxxx PEP005 (0.05%) PEP-005-018 — US Scenario KO1704 |
| Estimated | ||||||||||||||||||||||||
| Pass- | Estimated | |||||||||||||||||||||||
| Unit Cost | Fees | Through | Total Cost | |||||||||||||||||||||
| Services | Unit | # Units | (AUS $) | (AUS $) | (AUS $) | (AUS $) | ||||||||||||||||||
| A. Study Management | ||||||||||||||||||||||||
°
|
Project Director — Americas | Days | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Project Administrative Support/Coordination — Americas (includes support for all functional areas) | Days | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Study Management | $ | **** | $ | **** | $ | **** | ||||||||||||||||||
| Zone Legend | ||||||||||||||||||||||||
| > Zone 1: USA | ||||||||||||||||||||||||
| B. Clinical Trial Initiation | ||||||||||||||||||||||||
| ° | Study Master File | All assumes using OCR SOPs. If using Sponsor SOPs, is custom priced | ||||||||||||||||||||||
| >Americas: **** sites for **** months | Site Months | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Regulatory Document Collection |
|||||||||||||||||||||||
| > Zone 1 | Site | **** | **** | **** | **** | **** | ||||||||||||||||||
| Central IRB (pass-throughs are estimates only; actual fees will be billed based on specific IRB fees) | Site | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Protocol Amendments — excluding ICF change (US sites only) |
|||||||||||||||||||||||
| > Xxxx 0 | Xxxxxxxxxx/xxxx | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Protocol Amendments — including ICF change (US sites only) |
|||||||||||||||||||||||
| > Xxxx 0 | Xxxxxxxxxx/xxxx | **** | **** | **** | **** | **** | ||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 7 |
°
|
Investigator Agreement Negotiation — Standard | |||||||||||||||||||||||
| >Americas | Site | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Int’l | Site | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Investigator Agreement Negotiation — Complex | |||||||||||||||||||||||
| >Americas | Site | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Investigator Agreement Amendments — Simple | |||||||||||||||||||||||
| >Americas | Amendments/site | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Investigator Agreement Amendments — Standard | |||||||||||||||||||||||
| >Americas | Amendments/site | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Investigator Agreement Amendments — Complex | |||||||||||||||||||||||
| >Americas | Amendments/site | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Letters of Indemnification(US sites only) | Site | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Facility Letters | |||||||||||||||||||||||
| ># Standard Facility Letters |
Site | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Notice Letters | Site | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
IND Safety Report (US sites only) |
|||||||||||||||||||||||
| > Xxxx 0 | Xxxxxxx/Xxxx | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Investigator Brochure Updates |
|||||||||||||||||||||||
| > Zone 1 | IB Updates/Site | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Collect Financial Disclosure at Site Closeout |
|||||||||||||||||||||||
| > Zone 1 | Site | **** | **** | **** | **** | **** | ||||||||||||||||||
| Sub-Total Clinical Trial Initiation |
$ | **** | $ | **** | $ | **** | ||||||||||||||||||
| C. Clinical Trial Management | ||||||||||||||||||||||||
° |
Clinical Trial Manager — Americas |
Days | **** | **** | **** | **** | **** | |||||||||||||||||
° |
Clinical Monitoring | |||||||||||||||||||||||
| >Site Initiation Visit | ||||||||||||||||||||||||
| >Zone 1: **** hrs on-site, ****hrs for prep, follow-up, and reports, and **** hrs for travel | Visits | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Site Interim Visits | ||||||||||||||||||||||||
| >Zone 1: assume **** visits/site, **** hrs on-site, **** hrs for prep, follow-up, and reports, and **** hrs for travel | Visits | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Site Close-Out Visit | ||||||||||||||||||||||||
| >Zone 1: **** hrs on-site, **** hrs for prep, follow-up, and reports, and **** hrs for travel | Visits | **** | **** | **** | **** | **** | ||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 8 |
°
|
Site Maintenance for **** hrs/month/site (**** sites x **** enrollment and treatment months) | |||||||||||||||||||||||
| >Zone 1:**** sites | Site Months | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
CRA Monthly Teleconferences |
|||||||||||||||||||||||
| >Zone 1:**** CRAs | Months | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Project Team Training | |||||||||||||||||||||||
| Project Director — Americas |
Hours | **** | **** | **** | **** | **** | ||||||||||||||||||
| CTM — Americas | Hours | **** | **** | **** | **** | **** | ||||||||||||||||||
| CRA Attendees | Hours | |||||||||||||||||||||||
| > Zone 1 CRAs: **** CRAs | Hours | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Clinical Grants Administration |
|||||||||||||||||||||||
| >Grant Payment Set-up | ||||||||||||||||||||||||
| >Zone 1 | Sites | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Grant Management (takes place for active sites from site set-up through **** days after site close-out; estimated amounts; actuals will be billed based on number of active sites set up) | ||||||||||||||||||||||||
| >Zone 1 | Site Quarters | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Payment Processing (estimated based on **** payments/site; actuals, including investigator and site related payment and any miscellaneous payments, actuals will be billed; pass through costs are related to photocopying and postage) | ||||||||||||||||||||||||
| >Zone 1: **** sites | Payments | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Estimated Investigator Grants | Enrolled Patient - Americas |
**** | **** | **** | ||||||||||||||||||||
°
|
Refund Checks (if needed; actuals will be billed) | Refund | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub Total Clinical Trial Management |
$ | **** | $ | **** | $ | **** | ||||||||||||||||||
| D. Safety and Medical | ||||||||||||||||||||||||
°
|
Medical Monitoring availability during business hours (flat fee of **** hours per month from first patient in through last patient out) | |||||||||||||||||||||||
| >Americas | Months | **** | **** | **** | **** | **** | ||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 9 |
| > Greater than **** hours per month will be billed at hourly rate (from first patient in through last patient out) (actuals will be billed) |
||||||||||||||||||||||||
| >Americas | Hours | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Medical Monitoring during Study Start Up and Study Close-out (actuals will be billed) | |||||||||||||||||||||||
| >Americas | Hours | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Safety Coordinator | |||||||||||||||||||||||
| >Americas — assumes **** day per month for **** months plus **** additional days for Safety Plan development | Days | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Safety Review of CRFs and Queries | Case | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Availability for receipt of SAEs during non-business hours (first patient in to last patient out plus one month) | |||||||||||||||||||||||
| >Americas | Month | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
SAE Reporting To Sponsor (with initial descriptive summary); | |||||||||||||||||||||||
| actuals will be billed | Report | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
**** | |||||||||||||||||||||||
| >Monthly Database Maintenance (first patient in to database close) | Month | **** | **** | **** | **** | **** | ||||||||||||||||||
| Sub-Total Safety and Medical | $ | **** | $ | **** | $ | **** | ||||||||||||||||||
| E. Clinical Data Management | ||||||||||||||||||||||||
°
|
Clinical Data Management Oversight — Int’l (assumes ****% FTE x **** months for Start-up, ****% FTE for **** months for Duration, and ****% FTE for **** months for Close-out) | Days | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Development of Data Management Plan (includes **** major and **** minor revision; add’l revisions will be billed at per diem rates) | Days | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
CRF Design (assumes **** page CRF, **** unique CRF pages; includes **** review cycles) | Project | **** | **** | **** | **** | **** | |||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 10 |
°
|
Database Development, Testing (assumes **** page CRF, **** unique CRF pages) |
Project | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Database Maintenance | Months | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Estimated Data Entry (actuals will be billed) |
|||||||||||||||||||||||
| >Pages in Int’l | Pages | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Estimated Data Review and Query Resolution (assumes **** issue per **** CRF pages, Queries will be billed on actuals. | |||||||||||||||||||||||
| > CRF Pages + Electronic data visits (eg labs, ABPM) |
Page | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Issues | Issue | **** | **** | **** | **** | **** | ||||||||||||||||||
| >Manual Checks | Check | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Clinical/Medical Review - Overall review of each case to ensure clinical/medical integrity. |
Page | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Quality Control Check of Database versus CRF of Safety & Efficacy Data for all Subjects (assumes **** CRF pages per patient) ****% of of pages | Page | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Quality Control Check of Database versus CRF - **** CRF data for square root of patients (assumes **** CRF pages per patient) - **** patients | Page | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
CRF Tracking and Filing (includes all ancillary pages; actuals will be billed) | Page | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Dictionary - ATC Coding of Medications (actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Dictionary Coding of Adverse Event terms to MedDRA (estimated to be **** per patient; actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Dictionary Coding of Medication Terms (estimated to be **** per patient; actuals will be billed) | Term | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Local Laboratory Data | |||||||||||||||||||||||
| >Database Setup | Project | **** | **** | **** | **** | **** |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 11 |
| >Local Normal Ranges Processed (actuals will be billed) |
Sets | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Reconciliation of the Safety and Clinical Database (actuals will be billed) | SAE | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Interim Database Lock | patients | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Loading of protocol deviations log | hours | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Clinical Data Management |
$ | **** | $ | **** | $ | **** | ||||||||||||||||||
| F. Biometrics Analysis | ||||||||||||||||||||||||
°
|
Biometrics Team Manager | Days | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Project Data Setup | Project | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Statistical Plan | Project | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Design of Table Shells (Mocks) | Project | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Mock Annotation | Project | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Programming of Data Displays (actuals will be billed) | |||||||||||||||||||||||
| Unique Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||
| Repeat Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||
| Unique Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||
| Repeat Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||
| Unique Figures | Figure/Graph | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Programming of Interim Data Displays (actuals will be billed) | |||||||||||||||||||||||
| Unique Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||
| Repeat Tables | Table | **** | **** | **** | **** | **** | ||||||||||||||||||
| Unique Listings | Listing | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Custom Derived Data Sets | |||||||||||||||||||||||
| >Initial (Assumes **** datasets) |
Project | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Programmatic Evaluability/Outcome |
|||||||||||||||||||||||
| >Regular or Advanced | Project | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Statistical Analysis | |||||||||||||||||||||||
| Project | **** | **** | **** | **** | **** | |||||||||||||||||||
°
|
Interim Analysis | |||||||||||||||||||||||
| >Regular | Interim Analysis | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Standard Data Transfer | |||||||||||||||||||||||
| >Initial | Transfer | **** | **** | **** | **** | **** | ||||||||||||||||||
°
|
Biometrics CRF review | hours | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Biometrics | $ | **** | $ | **** | $ | **** | ||||||||||||||||||
| G. Clinical Writing | All assumes using OCR SOPs. If using Sponsor SOPs/templates, all CW is custom priced | |||||||||||||||||||||||
°
|
Day **** Top Line Report | Report | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Clinical Writing |
$ | **** | $ | **** | $ | **** | ||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 12 |
| I. Regulatory Services | ||||||||||||||||||||||||
°
|
CRF Filing and Reconciliation (actuals will be billed) | Pages | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Regulatory Site Drug Release Approval |
# Sites | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Regulatory Services |
$ | **** | $ | **** | $ | **** | ||||||||||||||||||
| K. Clinical Supplies Management | ||||||||||||||||||||||||
°
|
General Pharmaceutics project management (Americas) x hours per month |
Month | **** | **** | $ | **** | $ | **** | $ | **** | ||||||||||||||
°
|
Receipt of Returned Drug Americas | Receipt | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Return of study drug to Sponsor or Certified Destructor (incl. provision of certificate of destruction) | Destruction run | **** | **** | **** | **** | **** | |||||||||||||||||
°
|
Final reconciliation (Americas) |
Day | **** | **** | **** | **** | **** | |||||||||||||||||
| Sub-Total Clinical Supplies Management |
$ | **** | $ | **** | $ | **** | ||||||||||||||||||
| Estimated Services Budget | $ | **** | ||||||||||||||||||||||
| Estimated Pass Through | $ | **** | ||||||||||||||||||||||
| Total Estimated Budget | $ | **** | ||||||||||||||||||||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 13 |
| • | Travel | ||
| • | Delivery Costs | ||
| • | Printing or copying costs | ||
| • | Meeting costs | ||
| • | Telecommunication Costs (which may include telephone, fax, paper, conference calls, or PC connectivity charges) | ||
| • | All other Project related expenses that are not related to service fees |
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 14 |
Westpac Banking Corporation,
Xxxxxxxx Xxxxxxxx Xxxxxx,
Xxxxx Xxxx, XXX
SWIFT ID: ****
BSB/Account Number: ****
| Xxxxxx Operations Pty Ltd. | Omnicare CR, Inc. | |||||
BY:
|
/s/ Xxxxxx Xxxxx | BY: | /s/ Xxxx Xxxxx, PhD | |||
Name:
|
Xxxxxx Xxxxx | Name: | Xxxx Xxxxx, PhD | |||
Title:
|
CFO & VP, Finance and Operations | Title: | Chief Executive Officer | |||
Date:
|
Date: | |||||
| **** | Certain confidential information contained in this document, marked with four asterisks, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| Page 15 |
